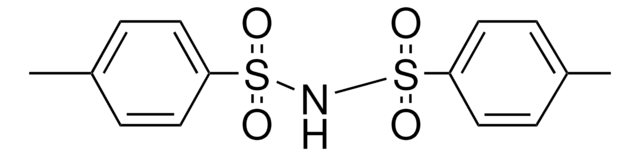
236330
p-Toluenesulfonamide
ReagentPlus®, ≥99%
Synonym(s):
4-Methylbenzene-1-sulfonamide, p-Tosylamide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3C6H4SO2NH2
CAS Number:
Molecular Weight:
171.22
Beilstein:
472689
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
≥99%
form
solid
mp
134-137 °C (lit.)
SMILES string
Cc1ccc(cc1)S(N)(=O)=O
InChI
1S/C7H9NO2S/c1-6-2-4-7(5-3-6)11(8,9)10/h2-5H,1H3,(H2,8,9,10)
InChI key
LMYRWZFENFIFIT-UHFFFAOYSA-N
Gene Information
human ... CA1(759) , CA2(760) , CA5A(763) , CA5B(11238)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Cyanomethylenetributylphosphorane (CMBP)-mediated reaction of p-toluenesulfonamide with alcohols has been investigated.
Application
p-Toluenesulfonamide has been employed:
- as nucleophile during tetrabutylammonium fluoride (TBAF) catalyzed vinyl aziridine opening reaction
- as reagent during selective aziridination of olefins catalyzed by dirhodium (II) caprolactamate
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
395.6 °F - closed cup
Flash Point(C)
202 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Arthur J Catino et al.
Organic letters, 7(13), 2787-2790 (2005-06-17)
[reaction: see text] A mild, efficient, and selective aziridination of olefins catalyzed by dirhodium(II) caprolactamate [Rh(2)(cap)(4).2CH(3)CN] is described. Use of p-toluenesulfonamide (TsNH(2)), N-bromosuccinimide (NBS), and potassium carbonate readily affords aziridines in isolated yields of up to 95% under extremely mild
E Simone et al.
Lab on a chip, 18(15), 2235-2245 (2018-06-28)
In this work, a novel multi-microfluidic crystallization platform called MMicroCryGen is presented, offering a facile methodology for generating individual crystals for fast and easy screening of the polymorphism and crystal habit of solid compounds. The MMicroCryGen device is capable of
Mitsunobu-type alkylation of p-toluenesulfonamide. A convenient new route to primary and secondary amines.
Tsunoda T, et al.
Tetrahedron Letters, 37(!4), 2457-2458 (1996)
Charles H Reynolds et al.
Journal of medicinal chemistry, 51(8), 2432-2438 (2008-04-03)
Ligand efficiency (i.e., potency/size) has emerged as an important metric in drug discovery. In general, smaller, more efficient ligands are believed to have improved prospects for good drug properties (e.g., bioavailability). Our analysis of thousands of ligands across a variety
Yang Yang et al.
Organic letters, 13(20), 5608-5611 (2011-09-16)
BF(3)·OEt(2)-catalyzed direct cyanation of indoles and pyrroles using a less toxic, bench-stable, and easily handled electrophilic cyanating agent N-cyano-N-phenyl-para-toluenesulfonamide (NCTS) affords 3-cyanoindoles and 2-cyanopyrroles in good yields with excellent regioselectivity. The substrate scope is broad with respect to indoles and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service