236241
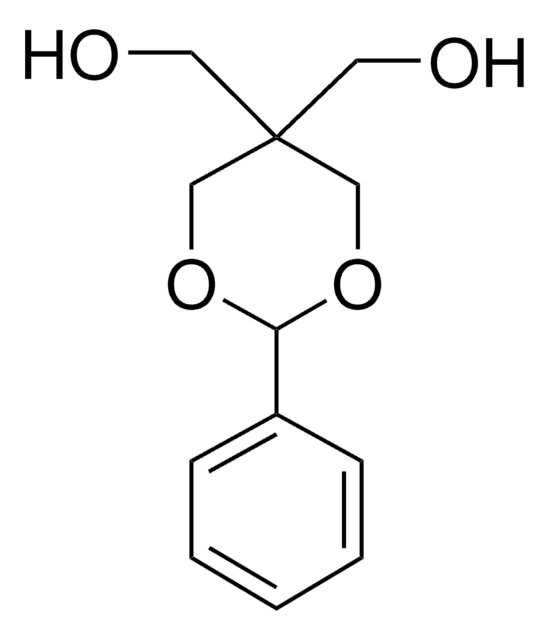
Pentaerythritol
99%
Synonym(s):
2,2-Bis(hydroxymethyl)-1,3-propanediol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C(CH2OH)4
CAS Number:
Molecular Weight:
136.15
Beilstein:
1679274
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
vapor pressure
<1 mmHg ( 20 °C)
Quality Level
Assay
99%
bp
276 °C/30 mmHg (lit.)
mp
253-258 °C (lit.)
SMILES string
OCC(CO)(CO)CO
InChI
1S/C5H12O4/c6-1-5(2-7,3-8)4-9/h6-9H,1-4H2
InChI key
WXZMFSXDPGVJKK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Pentaerythritol is acrystalline , odour less white solid. The presence of neopentane core and four terminalhydroxyl groups makes it a versatile substrate for the synthesispolyfunctionalized compounds.
Application
Pentaerythritol can be used as a starting material to prepare four armed polyurethane prepolymers, which are applicable in the synthesis of UV cured polyurethane dispersions.
It can be used as an additive in the preparation of flame retardant polymers.
It can also be used as a precursor to synthesize polyethylene glycol/4,4′-diphenylmethane diisocyanate/pentaerythritol copolymer which is applicable as heat storage material.
It can be used as an additive in the preparation of flame retardant polymers.
It can also be used as a precursor to synthesize polyethylene glycol/4,4′-diphenylmethane diisocyanate/pentaerythritol copolymer which is applicable as heat storage material.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chin-Wen Chen et al.
Polymers, 12(9) (2020-09-10)
Bio-based unsaturated poly(butylene adipate-co-butylene itaconate) (PBABI) aliphatic copolyesters were synthesized with pentaerythritol (PE) as a modifier, observing the melting point, crystallization, and glass transition temperatures were decreased from 59.5 to 19.5 °C and 28.2 to -9.1 °C as an increase
Zhiming Zhang et al.
Organic & biomolecular chemistry, 9(24), 8220-8223 (2011-10-13)
A trimer of cholic acid made by linking three cholic acid molecules with pentaerythritol via click chemistry serves as the center of an artificial metallohydrolase after the complexation of the triazole groups with a Zn(2+) ion. The invertible amphiphilic cavity
Mohamed Touaibia et al.
Chemical communications (Cambridge, England), (4)(4), 380-382 (2007-01-16)
Several oligomannoside clusters having a hundred-fold increase in affinities toward E. coli were synthesized by Cu(I)-catalyzed [1,3]-dipolar cycloadditions using pentaerythritol scaffolds bearing either alkyne or azide functionalities.
Tao Tu et al.
The Journal of organic chemistry, 73(14), 5255-5263 (2008-06-19)
Molecular scaffolds that have well-defined geometries, are easy to synthesize and functionalize, and can hold attached sites of molecular recognition in suitable orientations are useful tools in various areas of science and technology. The utility of the tetraphenyl ether of
Samar Hamdy et al.
Journal of pharmaceutical and biomedical analysis, 44(4), 914-923 (2007-06-26)
The present study had two main objectives. First, was to compare the immune stimulatory effect of two synthetic lipid A analogues (7-acyl lipid A and pentaerythritol-based lipid A (PET lipid A)) on maturation/stimulation of bone marrow derived dendritic cells (DCs).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service