234524
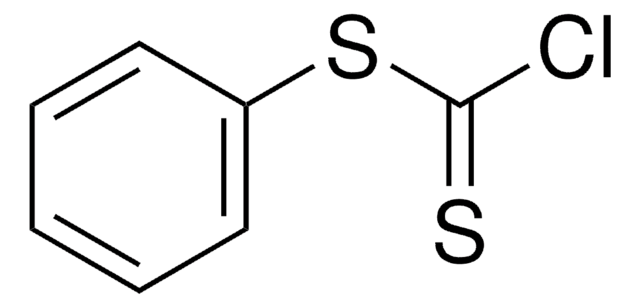
O-Phenyl chlorothionoformate
99%
Synonym(s):
Phenyl thionochloroformate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ClC(S)OC6H5
CAS Number:
Molecular Weight:
172.63
Beilstein:
774830
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.581 (lit.)
bp
81-83 °C/6 mmHg (lit.)
density
1.248 g/mL at 25 °C (lit.)
functional group
chloro
phenoxy
SMILES string
ClC(=S)Oc1ccccc1
InChI
1S/C7H5ClOS/c8-7(10)9-6-4-2-1-3-5-6/h1-5H
InChI key
KOSYAAIZOGNATQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
O-Phenyl chlorothionoformate has been used in:
- stereodirected synthesis of optically active, (−)-mintlactone
- synthesis of peptide α-thioesters having a variety of C-terminal amino acids
- synthesis of scyllo-inositol derivatives
- thionocarbonylation of unprotected thymine nucleosides
- preparation of phenoxythiocarbonyl esters of protected ribonucleosides
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
177.8 °F - closed cup
Flash Point(C)
81 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Canadian Journal of Chemistry, 71, 186-186 (1993)
Total synthesis of (-)-mintlactone.
Carda M and Marco JA.
Tetrahedron Letters, 32(38), 5191-5192 (1991)
A synthetic approach to a peptide α-thioester from an unprotected peptide through cleavage and activation of a specific peptide bond by N-acetylguanidine.
Ryo Okamoto et al.
Angewandte Chemie (International ed. in English), 51(1), 191-196 (2011-11-19)
Synlett, 221-221 (1993)
Yedi Sun et al.
Bioorganic & medicinal chemistry, 16(15), 7177-7184 (2008-07-19)
scyllo-Inositol has shown promise as a potential therapeutic for Alzheimer's disease, by directly interacting with the amyloid beta (Abeta) peptide to inhibit Abeta42 fiber formation. To explore the molecular details of the inositol-Abeta42 interaction, a series of scyllo-inositol derivatives have
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) >95% in F+ active](/deepweb/assets/sigmaaldrich/product/structures/206/487/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d/640/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d.png)




