208795
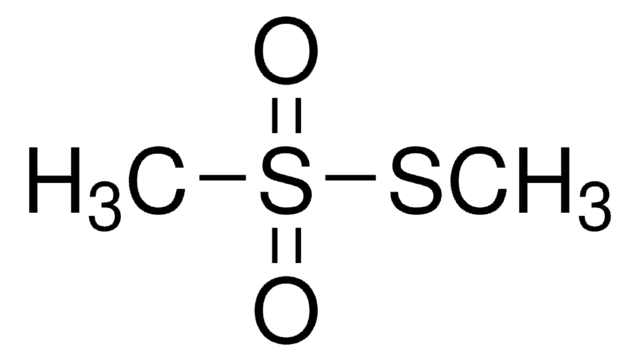
S-Methyl methanethiosulfonate
97%
Synonym(s):
S-Methyl thiomethanesulfonate, MMTS
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3SO2SCH3
CAS Number:
Molecular Weight:
126.20
Beilstein:
1446059
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.513 (lit.)
bp
69-71 °C/0.4 mmHg (lit.)
solubility
DMF: soluble 1:1
chloroform: soluble 200 ul per mL, clear, faintly yellow-green
water: soluble 1:5
density
1.337 g/mL at 25 °C (lit.)
functional group
disulfide
SMILES string
CSS(C)(=O)=O
InChI
1S/C2H6O2S2/c1-5-6(2,3)4/h1-2H3
InChI key
XYONNSVDNIRXKZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
S-Methyl methanethiosulfonate is a reagent used to synthesize thiols and thioesters. Used as cross-linking agent to prepare polymer networks. Interaction of S-methyl methanethiosulfonate (MMTS) with dipalmitoylphosphatidylcholine (DPPC) bilayers has been investigated by FTIR and surface-enhanced Raman spectroscopy.
Application
S-Methyl methanethiosulfonate was used as sulfenylating agent for β-keto sulfoxides, methylene compounds, half-esters of malonic acids and aryl Grignard reagents. It was also used as a reagent to trap the natural thiol-disulfide state of the proteins.
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
188.6 °F - closed cup
Flash Point(C)
87 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Journal of the Chemical Society. Perkin Transactions 1, 3167-3167 (1993)
Sustainable Three-Component Synthesis of Isothioureas from Isocyanides, Thiosulfonates, and Amines
P Mampuys, et al.
Angewandte Chemie (International Edition in English), 126 (2014)
Synthetic Communications, 22, 1359-1359 (1992)
The Journal of Organic Chemistry, 58, 6132-6132 (1993)
Sustainable Three-Component Synthesis of Isothioureas from Isocyanides, Thiosulfonates, and Amines
P Mampuys, et al.
Angewandte Chemie (International Edition in English), 126, 13063-13068 (2014)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service