196665
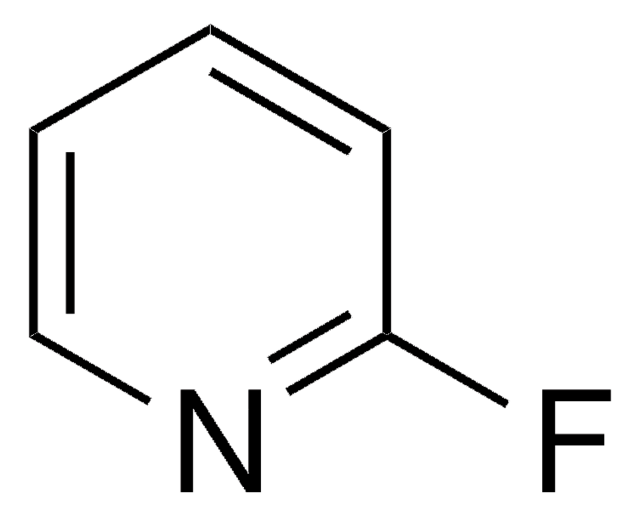
3-Fluoropyridine
99%
Synonym(s):
3-Fluoropyridine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
FC5H4N
CAS Number:
Molecular Weight:
97.09
Beilstein:
1363666
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.472 (lit.)
bp
107-108 °C (lit.)
density
1.13 g/mL at 25 °C (lit.)
functional group
fluoro
SMILES string
Fc1cccnc1
InChI
1S/C5H4FN/c6-5-2-1-3-7-4-5/h1-4H
InChI key
CELKOWQJPVJKIL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3-Fluoropyridine is oxidized to corresponding N-oxides, which on treatment with hot acetic anhydride and hydrolysis yields 3-halo-2-pyridones. Microwave spectrum of 3-fluoropyridine has been investigated in the frequency range of 8-18GHz at dry ice temperature. Deprotonation of 3-fluoropyridine with Bu3MgLi in THF at -10°C has been studied.
Application
3-Fluoropyridine was used in the synthesis of 3-halopyridin-4-yl-boronic acids and esters.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
55.4 °F - closed cup
Flash Point(C)
13 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Microwave spectrum, dipole moment, quadrupole coupling constants, and centrifugal distortion constants of 3-fluoropyridine.
Sharma SD and Doraiswamy S.
Journal of Molecular Spectroscopy, 59(2), 216-225 (1976)
Synthesis of novel halopyridinylboronic acids and esters. Part 3: 2, or 3-Halopyridin-4-yl-boronic acids and esters.
Bouillon A, et al.
Tetrahedron, 58(22), 4369-4373 (2002)
Pyridine Derivatives. III. The Rearrangement of Some Simple 3-Halopyridine-N-oxides1.
Cava MP and WEINSTEIN B.
The Journal of Organic Chemistry, 23(11), 1616-1617 (1958)
Deprotonation of fluoro aromatics using lithium magnesates.
Awad H, et al.
Tetrahedron Letters, 45(36), 6697-6701 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service