All Photos(1)
About This Item
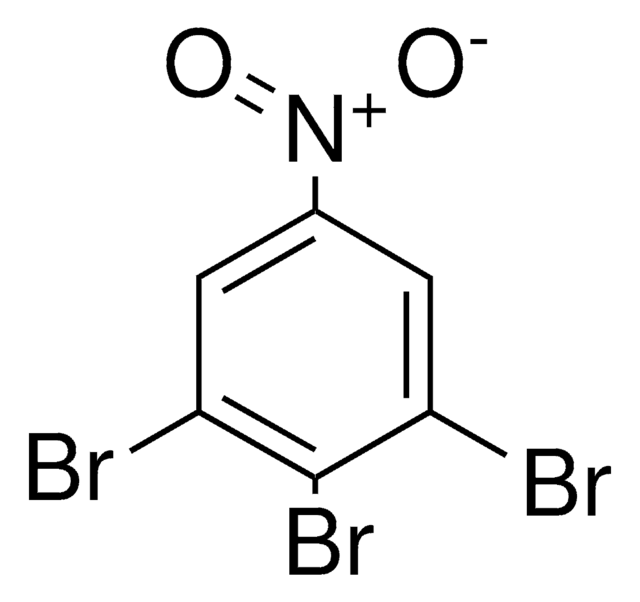
Linear Formula:
Br2C6H2(NO2)NH2
CAS Number:
Molecular Weight:
295.92
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97%
mp
205-207 °C (lit.)
functional group
bromo
nitro
SMILES string
Nc1c(Br)cc(cc1Br)[N+]([O-])=O
InChI
1S/C6H4Br2N2O2/c7-4-1-3(10(11)12)2-5(8)6(4)9/h1-2H,9H2
InChI key
YMZIFDLWYUSZCC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2,6-Dibromo-4-nitroaniline has been used in the synthesis of:
- blue disperse dyes
- 7-bromo-5-iodo-4-oxo-1,4-dihydroquinoline-2-carboxylic acid
- diphenols-amides such as N-(2,6-dibromo-4-nitrophenyl)-4,4-bis(4-hydroxyphenyl)-pentanamide and N-(2,6-diiodo-4-nitrophenyl)-4,4-bis(4-hydroxyphenyl)-pentanamide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
K Suresh Kumar et al.
Environmental monitoring and assessment, 127(1-3), 67-72 (2006-08-10)
Facile, selective and sensitive spectrophotometric method has been developed for the determination of bendiocarb in its insecticidal formulations, fortified water, food grains, agriculture wastewater and agriculture soil samples with prepared reagents. The method was based on alkaline hydrolysis of the
Polymerization by phase transfer catalysis. 24. Synthesis of condensation polymers derived from halogenated diphenol-amides with the amide group in the side chain.
Tagle LH, et al.
Polym. Bull., 42(6) (1999)
Substituent effects on the colour, dyeing and fastness properties of 4-phenylazo-1-naphthylamines and of 2-acetylamino-5-methoxy-4-N-?-cyanoethyl-N-?-hydroxyethylaminoazobenzene.
Peters AT.
Journal of the Society of Dyers and Colourists, 104(9), 344-348 (1988)
Synthesis of 7-Bromo-5-iodo-4-oxo-1, 4-dihydroquinoline-2-carboxylic acid.
Dumont F and Slegers G.
Bull. Soc. Chim. Belg., 104(8), 505-507 (1995)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![Calix[4]arene-25,26,27,28-tetrol 95%](/deepweb/assets/sigmaaldrich/product/structures/198/765/9972559b-b50f-4745-b1fd-9a468e47ef55/640/9972559b-b50f-4745-b1fd-9a468e47ef55.png)




