186511
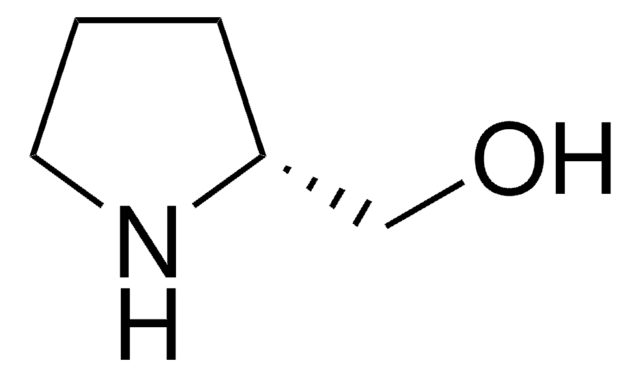
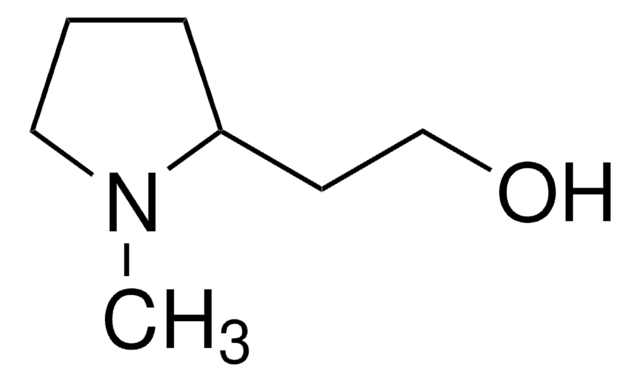
(S)-(+)-2-Pyrrolidinemethanol
97%
Synonym(s):
(S)-(+)-2-(Hydroxymethyl)pyrrolidine, L-Prolinol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H11NO
CAS Number:
Molecular Weight:
101.15
Beilstein:
79843
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
optical activity
[α]20/D +31°, c = 1 in toluene
refractive index
n20/D 1.4853 (lit.)
bp
74-76 °C/2 mmHg (lit.)
density
1.025 g/mL at 25 °C (lit.)
functional group
hydroxyl
SMILES string
OC[C@@H]1CCCN1
InChI
1S/C5H11NO/c7-4-5-2-1-3-6-5/h5-7H,1-4H2/t5-/m0/s1
InChI key
HVVNJUAVDAZWCB-YFKPBYRVSA-N
Related Categories
General description
(S)-(+)-2-Pyrrolidinemethanol, also known as (S)-(+)-prolinol is a chiral building block that is used for the synthesis of chiral organic compounds. It is also used as a chiral auxiliary in asymmetric synthesis and a chiral ligand in asymmetric catalysis.
Application
(S)-(+)-2-Pyrrolidinemethanol can be used as a starting material for the synthesis of (S)-α, α-diaryl-2-pyrrolidinemethanols. It plays an important role in this synthesis by providing chirality to the final product.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
186.8 °F - closed cup
Flash Point(C)
86 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Magnus Rueping et al.
Organic & biomolecular chemistry, 10(30), 6201-6210 (2012-05-16)
A highly efficient route for the synthesis of valuable 3,4-substituted chromenone derivatives by the reaction of 1,3-diketones with aldehydes in the presence of l-proline was developed. The reactions take advantage of readily available starting materials and follow a Knoevenagel condensation/Michael
Janine Cossy
Chemical record (New York, N.Y.), 5(2), 70-80 (2005-04-13)
The synthesis of optically active substituted piperidines has been achieved by using four different methodologies. The first one is an intramolecular nucleophilic displacement of activated alcohol moieties that was used to build up the piperidine ring of (-)-prosophylline and (-)-slaframine
Yuko Kawanami et al.
The Journal of organic chemistry, 74(20), 7908-7921 (2009-09-19)
The photochirogenesis of 2-anthracenecarboxylic acid (AC) complexed to a hydrogen-bonding template (TKS159) was investigated to obtain mechanistic information on how chirogenesis is achieved for the dimerization of AC. Complexation of AC to TKS159 leads to the shielding of one of
Anna Maria Deobald et al.
Organic & biomolecular chemistry, 10(38), 7681-7684 (2012-08-25)
An eco-friendly synthesis of highly functionalized epoxides and their incorporation into an organocatalytic multicomponent approach are reported. For this, a modified class of diarylprolinol silyl ethers was designed to enable high catalytic activity in an environmentally benign solvent system. The
Chuan Wang et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(37), 11531-11535 (2012-07-28)
Sulfenylated oxindoles: The first asymmetric sulfenylation of N-Boc-protected oxindoles has been developed to provide products containing a tetrasubstituted stereogenic center in high to excellent yields (86-98 %) and, in most cases, excellent enantioselectivities (up to 96 % ee; see scheme).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service