186481
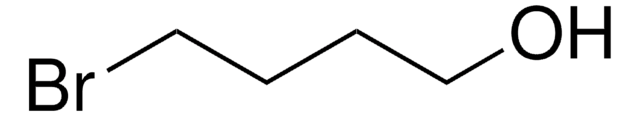
6-Bromo-1-hexanol
97%
Synonym(s):
Hexamethylene bromohydrin
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
Br(CH2)6OH
CAS Number:
Molecular Weight:
181.07
Beilstein:
1732415
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
reaction suitability
reagent type: cross-linking reagent
refractive index
n20/D 1.482 (lit.)
bp
105-106 °C/5 mmHg (lit.)
density
1.384 g/mL at 25 °C (lit.)
functional group
bromo
hydroxyl
storage temp.
2-8°C
SMILES string
BrCCCCCCO
InChI
1S/C6H13BrO/c7-5-3-1-2-4-6-8/h8H,1-6H2
InChI key
FCMCSZXRVWDVAW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
6-Bromo-1-hexanol, also known as hexamethylene bromohydrin is a cross-linking reagent that is commonly used in organic and polymeric synthesis.
Application
6-Bromo-1-hexanol is used as a cross linker to synthesize cyclic, branched, and bicyclic oligonucleotides by click chemistry. It is also utilized to synthesize gel polymer electrolytes, and polymerizable ionic liquids (PILs) such as 1-[(6-methacryloyloxy)hexyl]3-methylimidazolium bromide.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cycling performance of lithium polymer cells assembled by in situ polymerization of a non-flammable ionic liquid monomer
YS Lee
Electrochimica Acta, 106, 460-464 (2013)
New strategies for cyclization and bicyclization of oligonucleotides by click chemistry assisted by microwaves
J Lietard
The Journal of Organic Chemistry, 73, 191-200 (2008)
Synthesis of Polymerizable Ionic Liquid Monomer and Its Characterization
J Sardar
IOP Conference Series: Materials Science and Engineering, 111, 012021-012021 (2016)
Zrinka Rajic et al.
Journal of inorganic biochemistry, 169, 50-60 (2017-01-29)
We disclose here the studies that preceded and guided the preparation of the metal-based, redox-active therapeutic Mn(III) meso-tetrakis(N-n-butoxyethylpyridyl)porphyrin, MnTnBuOE-2-PyP
Eike T Röchow et al.
Polymers, 12(8) (2020-08-06)
Solid polymer electrolytes for bipolar lithium ion batteries requiring electrochemical stability of 4.5 V vs. Li/Li+ are presented. Thus, imidazolium-containing poly(ionic liquid) (PIL) networks were prepared by crosslinking UV-photopolymerization in an in situ approach (i.e., to allow preparation directly on
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service