186090
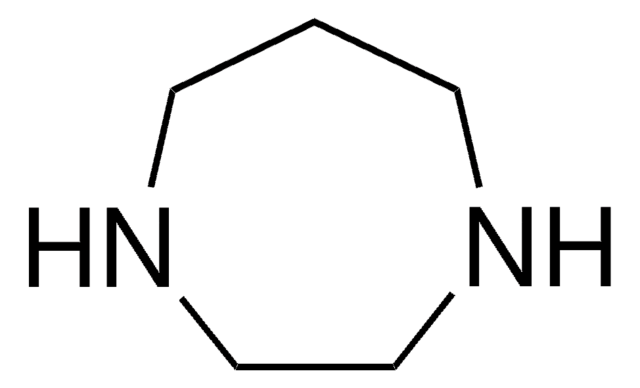
1-Methylhomopiperazine
97%
Synonym(s):
Hexahydro-1-methyl-1H-1,4-diazepine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H14N2
CAS Number:
Molecular Weight:
114.19
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.477 (lit.)
bp
74-75 °C/35 mmHg (lit.)
density
0.918 g/mL at 25 °C (lit.)
storage temp.
−20°C
SMILES string
CN1CCCNCC1
InChI
1S/C6H14N2/c1-8-5-2-3-7-4-6-8/h7H,2-6H2,1H3
InChI key
FXHRAKUEZPSMLJ-UHFFFAOYSA-N
General description
1-Methylhomopiperazine undergoes coupling reaction with a series of diazonium salts to afford the 4-methyl-1-[2-aryl-1-diazenyl]-1,4-diazepanes.
Application
1-Methylhomopiperazine was used in the synthesis of:
- novel benzimidazole derivatives having pharmacological activities
- platinum(II) substituted disulfide complexes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
113.0 °F - closed cup
Flash Point(C)
45 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis and characterization of a series of 4-methyl-1-[2-aryl-1-diazenyl]-1, 4-diazepanes and 1, 4-di-[2-aryl-1-diazenyl]-1, 4-diazepanes.
Moser SL and Vaughan K.
Canadian Journal of Chemistry, 82(!2), 1725-1735 (2004)
H Nakano et al.
Bioorganic & medicinal chemistry, 8(2), 373-380 (2000-03-18)
Novel benzimidazole derivatives were synthesized and their pharmacological activities were examined. These compounds showed a good suppressive action on histamine release from rat peritoneal mast cells produced by antigen-antibody reaction, an antagonistic action on guinea pig ileum contraction caused by
Homopiperazine platinum (II) complexes containing substituted disulfide groups: crystal structure of [Pt II(homopiperazine)(diphenylsulfide) Cl] NO3.
Ali MS, et al.
Polyhedron, 21(1), 125-131 (2002)
Son T Nguyen et al.
Bioorganic & medicinal chemistry, 23(9), 2024-2034 (2015-03-31)
Recently we described a novel pyranopyridine inhibitor (MBX2319) of RND-type efflux pumps of the Enterobacteriaceae. MBX2319 (3,3-dimethyl-5-cyano-8-morpholino-6-(phenethylthio)-3,4-dihydro-1H-pyrano[3,4-c]pyridine) is structurally distinct from other known Gram-negative efflux pump inhibitors (EPIs), such as 1-(1-naphthylmethyl)-piperazine (NMP), phenylalanylarginine-β-naphthylamide (PAβN), D13-9001, and the pyridopyrimidine derivatives. Here
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service