180246
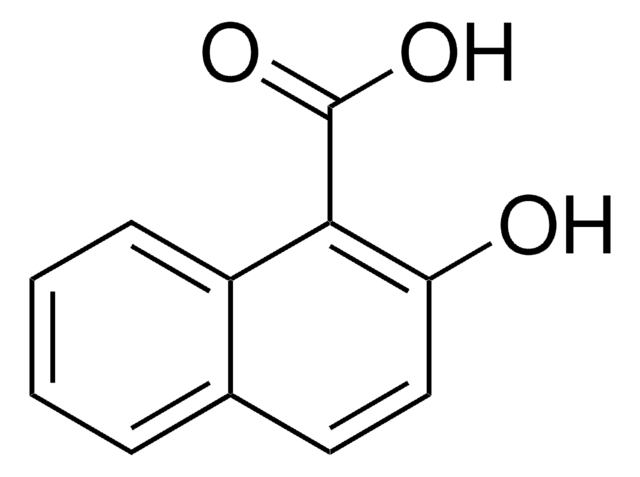
2-Naphthoic acid
98%
Synonym(s):
2-Naphthalenecarboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
C10H7CO2H
CAS Number:
Molecular Weight:
172.18
Beilstein:
972039
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
mp
185-187 °C (lit.)
solubility
alcohol: soluble
diethyl ether: soluble
hot water: slightly soluble
functional group
carboxylic acid
SMILES string
OC(=O)c1ccc2ccccc2c1
InChI
1S/C11H8O2/c12-11(13)10-6-5-8-3-1-2-4-9(8)7-10/h1-7H,(H,12,13)
InChI key
UOBYKYZJUGYBDK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Naphthoic acid (NPA) is a noncompetitive N-methyl-D-aspartate (NMDA) receptor inhibitor. The fluorescence spectra and electronic absorption of 2-naphthoic acid was studied.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Han Yu et al.
Molecular pharmacology, 84(4), 541-550 (2013-07-23)
N-Methyl-D-aspartate (NMDA) receptors mediate excitatory synaptic transmission in the central nervous system and play important roles in synaptic development and plasticity, but also mediate glutamate neurotoxicity. Recently, 2-naphthoic acid (NPA) and its derivatives have been identified as allosteric, noncompetitive NMDA
K G Mooney et al.
Journal of pharmaceutical sciences, 70(12), 1358-1365 (1981-12-01)
This study investigated the possible effects of simultaneous, noninstantaneous, reversible chemical ionization of carbon acids on the dissolution of a typical pharmaceutical carbon acid, phenylbutazone, and its deutero analog. The dissolution rate versus pH profile for phenylbutazone was consistent with
K L Yu et al.
Journal of medicinal chemistry, 39(12), 2411-2421 (1996-06-07)
In search for retinoic acid receptor (RAR) selective ligands, a series of 6-substituted 2-naphthoic acid retinoids were synthesized and evaluated in vitro in a transactivation assay and a competition binding assay for all RARs. These derivatives, in general, showed RAR
D P McNamara et al.
Journal of pharmaceutical sciences, 75(9), 858-868 (1986-09-01)
A mass transfer model was developed to describe the dissolution and reaction of acidic and basic compounds from a rotating disk in unbuffered water. Dissolution of two carboxylic acids, 2-naphthoic acid (1) and naproxen [(+)-6-methoxy-alpha-methyl-2-naphthaleneacetic acid, 2], and the free
An electronic spectral study of the influence of thermal and electronic processes on the determination of the excited singlet-state dissociation constants of 1-and 2-naphthoic acid.
Kovi PJ and Schulman SG.
Analytica Chimica Acta, 63(1), 39-52 (1973)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service