All Photos(1)
About This Item
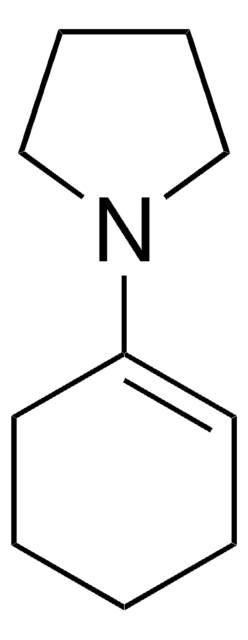
Linear Formula:
CH3COC6H9(=O)
CAS Number:
Molecular Weight:
140.18
Beilstein:
1858621
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.509 (lit.)
bp
111-112 °C/18 mmHg (lit.)
density
1.078 g/mL at 25 °C (lit.)
functional group
ketone
SMILES string
CC(=O)C1CCCCC1=O
InChI
1S/C8H12O2/c1-6(9)7-4-2-3-5-8(7)10/h7H,2-5H2,1H3
InChI key
OEKATORRSPXJHE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The keto-enol tautomerism of 2-acetylcyclohexanone (ACHE) in water was studied.
Application
2-Acetylcyclohexanone was used in the synthesis of anilinoethanolamines.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
174.2 °F - closed cup
Flash Point(C)
79 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Emilia Iglesias
The Journal of organic chemistry, 68(7), 2689-2697 (2003-03-29)
The kinetic study of the nitrosation of the enol of 2-acetylcyclohexanone (ACHE) has been performed in aqueous acid media in the absence and presence of alpha- and beta-cyclodextrin. The reaction is first-order with respect to both reactants concentration: [nitrite] and
Cédric Bouteiller et al.
Organic & biomolecular chemistry, 8(5), 1111-1120 (2010-02-19)
An operationally simple and concise synthesis of anilinoethanolamines, as NMDA NR2B receptor antagonist ifenprodil analogues, was developed via a copper-catalyzed amination of the corresponding bromoarene. Coupling was achieved with linear primary alkylamines, alpha,omega-diamines, hexanolamine and benzophenone imine, as well as
Emilia Iglesias
The Journal of organic chemistry, 68(7), 2680-2688 (2003-03-29)
The keto-enol tautomerism of 2-acetylcyclohexanone (ACHE) was studied in water under different experimental conditions. By contrast with other previously studied beta-diketones, the keto-enol interconversion in the ACHE system is a slow process. Under equilibrium conditions, the analysis of the absorbance
An alternative to the classical α-arylation: the transfer of an intact 2-iodoaryl from ArI(O₂CCF₃)₂.
Zhiyu Jia et al.
Angewandte Chemie (International ed. in English), 53(42), 11298-11301 (2014-09-10)
The α-arylation of carbonyl compounds is generally accomplished under basic conditions, both under metal catalysis and via aryl transfer from the diaryl λ(3)-iodanes. Here, we describe an alternative metal-free α-arylation using ArI(O2CCF3)2 as the source of a 2-iodoaryl group. The
Yoshihide Usami et al.
Molecules (Basel, Switzerland), 25(20) (2020-10-16)
Alkylamino coupling reactions at the C4 positions of 4-halo-1H-1-tritylpyrazoles were investigated using palladium or copper catalysts. The Pd(dba)2 catalyzed C-N coupling reaction of aryl- or alkylamines, lacking a β-hydrogen atom, proceeded smoothly using tBuDavePhos as a ligand. As a substrate
Articles
Buchwald phosphine ligands for C-C, C-N, and C-O bond formation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[4,4′-Bis(1,1-dimethylethyl)-2,2′-bipyridine] nickel (II) dichloride](/deepweb/assets/sigmaaldrich/product/structures/471/091/6faa29b1-bf8a-4d87-90b2-4cc55e082620/640/6faa29b1-bf8a-4d87-90b2-4cc55e082620.png)

![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)