177180
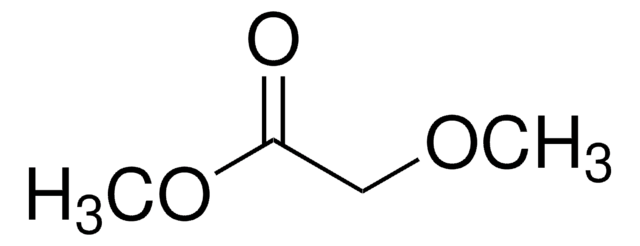
Methoxyacetone
95%
Synonym(s):
Methoxy-2-propanone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3COCH2OCH3
CAS Number:
Molecular Weight:
88.11
Beilstein:
1560175
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.397 (lit.)
bp
118 °C (lit.)
density
0.957 g/mL at 25 °C (lit.)
functional group
ether
ketone
SMILES string
COCC(C)=O
InChI
1S/C4H8O2/c1-4(5)3-6-2/h3H2,1-2H3
InChI key
CUZLJOLBIRPEFB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The gas-phase basicity of methoxyacetone was studied.
Application
Methoxyacetone was used in the synthesis of doubly borylated enolates.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
77.0 °F - closed cup
Flash Point(C)
25 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Atsushi Abiko et al.
Journal of the American Chemical Society, 124(36), 10759-10764 (2002-09-05)
The novel doubly borylated enolate is identified as an intermediate of the double aldol reaction of acetate esters. As a precursor to the formation of the doubly borylated enolate, carbon-bound boron enolates of carboxylic esters are spectroscopically characterized for the
Guy Bouchoux et al.
The journal of physical chemistry. A, 109(51), 11851-11859 (2005-12-22)
The gas-phase basicities of a representative set of hydroxy- and methoxycarbonyl compounds (hydroxyacetone, 1, 3-hydroxybutanone, 2, 3-hydroxy-3-methylbutanone, 3, 1-hydroxy-2-butanone, 4, 4-hydroxy-2-butanone, 5, 5-hydroxy-2-pentanone, 6, methoxyacetone, 7, 3-methoxy-2-butanone, 8, 4-methoxy-2-butanone, 9, and 5-methoxy-2-pentanone, 10) were experimentally determined by the equilibrium method
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service