165794
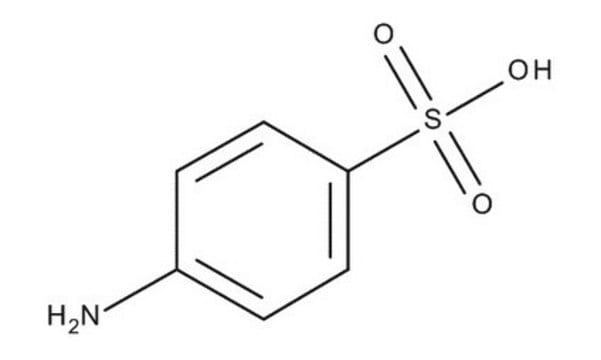
3-Aminobenzenesulfonic acid
97%
Synonym(s):
Metanilic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
H2NC6H4SO3H
CAS Number:
Molecular Weight:
173.19
Beilstein:
473264
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
powder
mp
>300 °C (lit.)
solubility
ethanol: very slightly soluble(lit.)
methanol: very slightly soluble(lit.)
functional group
sulfonic acid
SMILES string
Nc1cccc(c1)S(O)(=O)=O
InChI
1S/C6H7NO3S/c7-5-2-1-3-6(4-5)11(8,9)10/h1-4H,7H2,(H,8,9,10)
InChI key
ZAJAQTYSTDTMCU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3-Aminobenzenesulfonic acid undergoes microbial desulfonation to 3-aminophenol by Pseudomonas sp. strain S-313.
Application
3-Aminobenzenesulfonic acid was used in the synthesis of cytocompatible sulfonated polyanilines. It was used in fabrication of novel glucose biosensor having large active surface area and excellent conductivity.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Simon Doswald et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 27(12), 4108-4114 (2020-12-23)
The functionalization of magnetic nanoparticles has been an important field in the last decade due to the versatile applications in catalysis and biomedicine. Generally, a high degree of functionalities on the surface of the nanoparticles is desired. In this study
D Zürrer et al.
Applied and environmental microbiology, 53(7), 1459-1463 (1987-07-01)
Sulfur-limited batch enrichment cultures containing one of nine multisubstituted naphthalenesulfonates and an inoculum from sewage yielded several taxa of bacteria which could quantitatively utilize 19 sulfonated aromatic compounds as the sole sulfur source for growth. Growth yields were about 4
Yanyin Yang et al.
Macromolecular rapid communications, 32(12), 887-892 (2011-05-19)
We report here that by good design, polyaniline (PANI) can be cytocompatible and formed into usable scaffolds for bio-medical applications. By adjusting the ratio of two monomers, aniline (AN) and metanilic acid (MA), a series of copolymers with different sulfonation
Mei-Hwa Lee et al.
Biosensors & bioelectronics, 150, 111901-111901 (2019-11-27)
Molecularly imprinted polymers (MIPs) have been developed to replace antibodies for the recognition of target molecules (such as antigens), and have been integrated into electrochemical sensing approaches by polymerization onto an electrode. Electrochemical sensing is inexpensive and flexible, and has
Kai-Hsi Liu et al.
Biosensors, 10(3) (2020-03-04)
Molecularly imprinted polymers (MIPs) can often bind target molecules with high selectivity and specificity. When used as MIPs, conductive polymers may have unique binding capabilities; they often contain aromatic rings and functional groups, which can undergo π-π and hydrogen bonding
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service