163619
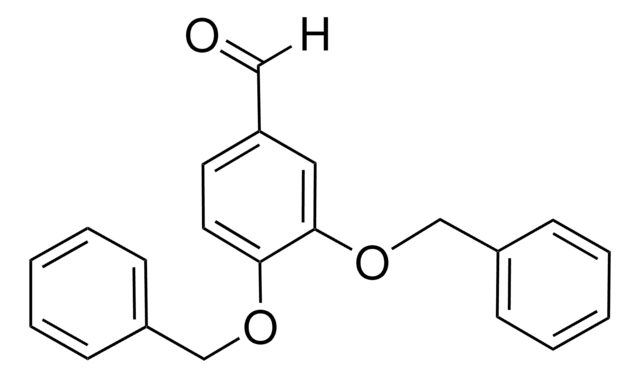
4-Benzyloxy-3-methoxybenzaldehyde
98%
Synonym(s):
O-Benzylvanillin, Vanillin benzyl ether
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH2OC6H3(OCH3)CHO
CAS Number:
Molecular Weight:
242.27
Beilstein:
1464258
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
62-64 °C (lit.)
functional group
aldehyde
phenyl
SMILES string
[H]C(=O)c1ccc(OCc2ccccc2)c(OC)c1
InChI
1S/C15H14O3/c1-17-15-9-13(10-16)7-8-14(15)18-11-12-5-3-2-4-6-12/h2-10H,11H2,1H3
InChI key
JSHLOPGSDZTEGQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Benzyloxy-3-methoxybenzaldehyde reacts with benzohydrazide to yield (E)-N′-(4-benzyloxy-3-methoxybenzylidene)benzohydrazide.
Application
4-Benzyloxy-3-methoxybenzaldehyde was used in the synthesis of 1,2-bis(4-benzyloxy-3-methoxyphenyl)-3-hydroxy-propionic acid. It was also used in first enantioselective total synthesis of a neurotrophic (-)-talaumidin.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of the erythro and threo forms of 1, 2-bis (4-hydroxy-3-methoxyphenyl)-l, 3-propanediol.
Berndtsson L, et al.
Acta Chemica Scandinavica. Series B, 34, 453-455 (1980)
(E)-N'-(4-Benzyloxy-3-methoxybenzylidene) benzohydrazide.
He Y-Z and Liu D-Z.
Acta Crystallographica Section E, Structure Reports Online, 61(11), o3855-o3856 (2005)
First enantioselective synthesis of (-)-talaumidin, a neurotrophic diaryltetrahydrofuran-type lignan.
Esumi T, et al.
Tetrahedron Letters, 47(24), 3979-3983 (2006)
C A Jackson et al.
Journal of applied microbiology, 122(4), 940-952 (2017-01-17)
The aim of this work was to isolate novel lignin-degrading organisms. Several pure cultures of bacteria that degrade lignin were isolated from bacterial consortia developed from decaying biomass. Among the isolates, Rhizobium sp. strain YS-1r (closest relative of Rhizobium petrolearium
Alla V Lipeeva et al.
European journal of medicinal chemistry, 100, 119-128 (2015-06-17)
A series of 2-(4-R-triazolyl)substituted 3-oxo-2,3-dihydrofurocoumarins have been synthesized by a regioselective cycloaddition of 2-azidooreoselone 1 or 2-azido-9-[(4-methylpiperazin-1-yl)methyl]oreoselone 2 with various alkynes in the presence of Cu(II)/ascorbate in water/methylene chloride reaction medium. The structure of 2-azidooreoselone was established by X-ray structure
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service