161497
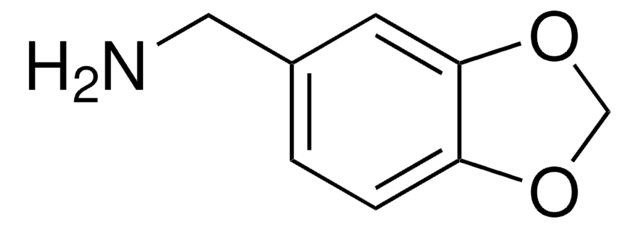
3,4-(Methylenedioxy)aniline
97%
Synonym(s):
5-Amino-1,3-benzodioxole
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C7H7NO2
CAS Number:
Molecular Weight:
137.14
Beilstein:
4919
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
bp
144 °C/16 mmHg (lit.)
mp
39-41 °C (lit.)
SMILES string
Nc1ccc2OCOc2c1
InChI
1S/C7H7NO2/c8-5-1-2-6-7(3-5)10-4-9-6/h1-3H,4,8H2
InChI key
XGNXYCFREOZBOL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3,4-(Methylenedioxy)aniline is an pharmaceutically important aniline derivative. It undergoes N-alkylation with cyclic secondary alkylamines in the presence of Shvo catalyst to yield N-arylpyrrolidines.
Application
3,4-(Methylenedioxy)aniline was used in the synthesis of γ-glutamylanilides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Rsoswsky et al.
Journal of medicinal chemistry, 22(9), 1034-1037 (1979-09-01)
Nine heretofore unknown mono- and dihydroxyanilide analogues of the cytotoxic mushroom metabolites L-glutamic acid gamma-(4-hydroxyanilide) (1) and L-glutamic acid gamma-(3,4-dihydroxyanilide) (3, agaridoxin) were synthesized and tested as inhibitors of the growth of B16 mouse melanoma cells in culture. The naturally
A general ruthenium-catalyzed synthesis of aromatic amines.
Dirk Hollmann et al.
Angewandte Chemie (International ed. in English), 46(43), 8291-8294 (2007-09-25)
K N Vennila et al.
Bioorganic chemistry, 81, 184-190 (2018-08-24)
The induced fit docking of anilino quinoline scaffold results in the required hydrogen bonding interactions with amino acid residues in the orthosteric site of 3 Phosphoinositide dependent kinase (PDK1). The rational design of 4-substituted amino quinolines is carried out and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service