All Photos(1)
About This Item
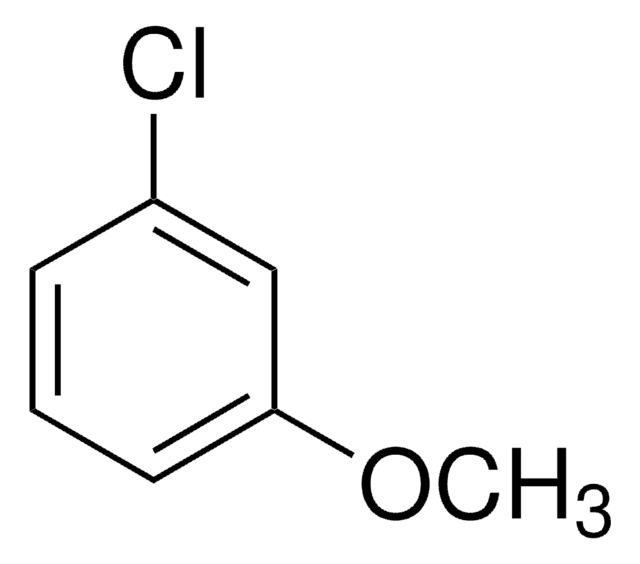
Linear Formula:
ClC6H4OCH3
CAS Number:
Molecular Weight:
142.58
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.545 (lit.)
bp
195-196 °C (lit.)
density
1.123 g/mL at 25 °C (lit.)
functional group
chloro
SMILES string
COc1ccccc1Cl
InChI
1S/C7H7ClO/c1-9-7-5-3-2-4-6(7)8/h2-5H,1H3
InChI key
QGRPVMLBTFGQDQ-UHFFFAOYSA-N
General description
2-Chloroanisole undergoes acetylation with acetic anhydride over large pore zeolites to give 4-acetyl-2-chloroanisole.
Application
2-Chloroanisole was used in headspace solid-phase microextraction method for the determination of haloanisoles in wine and spirit samples.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
168.8 °F - closed cup
Flash Point(C)
76 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Keith Smith et al.
Organic & biomolecular chemistry, 1(9), 1560-1564 (2003-08-21)
The acetylation of aryl ethers using acetic anhydride in the presence of zeolites under modest conditions in a solvent-free system gave the corresponding para-acetylated products in high yields. The zeolite can be recovered, regenerated and reused to give almost the
Natalia Campillo et al.
Journal of chromatography. A, 1210(2), 222-228 (2008-10-18)
A headspace solid-phase microextraction (HS-SPME) method for the determination of 12 haloanisoles in wine and spirit samples using gas chromatography with atomic emission detection (GC-AED) was developed. The different factors affecting the efficiency of the extraction were carefully optimized. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service