156965
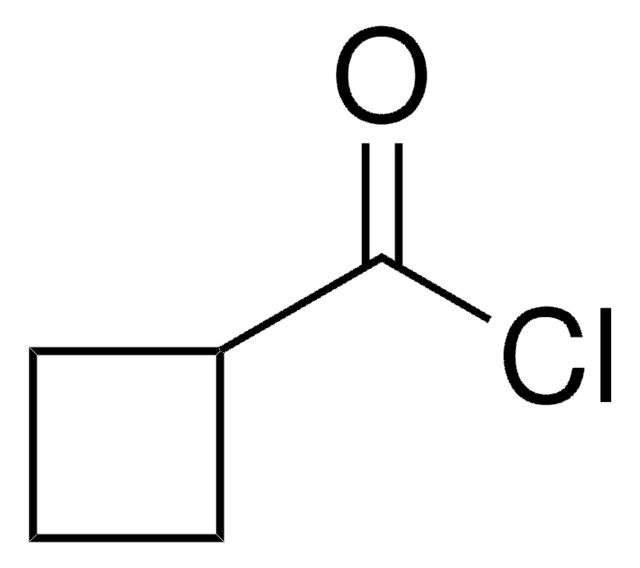
Cyclohexanecarbonyl chloride
98%
Synonym(s):
Hexahydrobenzoyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H11COCl
CAS Number:
Molecular Weight:
146.61
Beilstein:
742163
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.469 (lit.)
bp
184 °C (lit.)
density
1.096 g/mL at 25 °C (lit.)
functional group
acyl chloride
SMILES string
ClC(=O)C1CCCCC1
InChI
1S/C7H11ClO/c8-7(9)6-4-2-1-3-5-6/h6H,1-5H2
InChI key
RVOJTCZRIKWHDX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Electrochemical reduction of cyclohexanecarbonyl chloride at a hanging mercury drop electrode in acetonitrile has been reported.
Application
Cyclohexanecarbonyl chloride was used in the preparation of five thiourea derivative ligands having anti-bacterial and anti-yeast activity:
- (N-(diethylcarbamothioyl)cyclohexanecarboxamide
- N-(di-n-propylcarbamothioyl)cyclohexanecarboxamide
- di-n-butylcarbamothioyl)cyclohexanecarboxamide
- N-(diphenylcarbamothioyl)cyclohexanecarboxamide
- N-(morpholine-4-carbonothioyl)cyclohexanecarboxamide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
150.8 °F
Flash Point(C)
66 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hakan Arslan et al.
Molecules (Basel, Switzerland), 14(1), 519-527 (2009-01-27)
Five thiourea derivative ligands and their Ni(2+) and Cu(2+) complexes have been synthesized. The compounds were screened for their in vitro anti-bacterial activity using Gram-positive bacteria (two different standard strains of Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus faecalis, Streptococcus pyogenes, Bacillus
Electrochemical reduction of cyclohexanecarbonyl chloride at mercury cathodes in acetonitrile.
Urove GA and Peters DG.
Journal of the Electrochemical Society, 140(4), 932-935 (1993)
Shigeo Hayashi et al.
Journal of enzyme inhibition and medicinal chemistry, 29(6), 846-867 (2014-02-13)
Because of the pivotal role of cyclooxygenase (COX) in the inflammatory processes, non-steroidal anti-inflammatory drugs (NSAIDs) that suppress COX activities have been used clinically for the treatment of inflammatory diseases/syndromes; however, traditional NSAIDs exhibit serious side-effects such as gastrointestinal damage
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service