All Photos(3)
About This Item
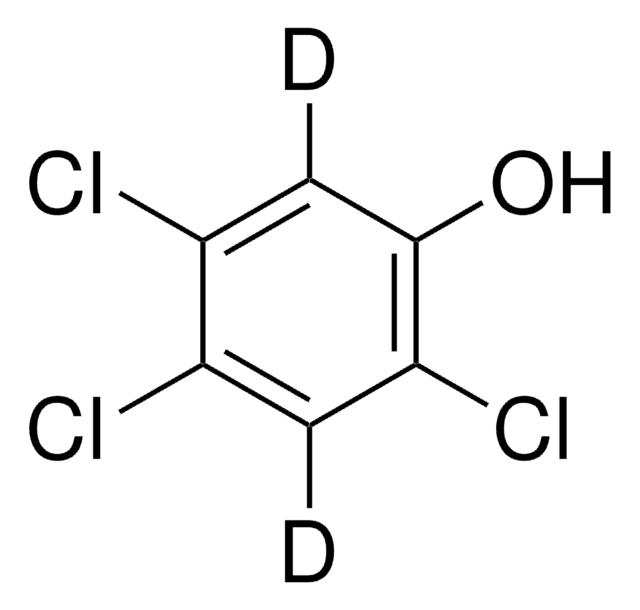
Linear Formula:
Cl3C6H2OH
CAS Number:
Molecular Weight:
197.45
Beilstein:
607569
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
bp
248 °C/740 mmHg (lit.)
mp
67-69 °C (lit.)
functional group
chloro
SMILES string
Oc1cc(Cl)c(Cl)cc1Cl
InChI
1S/C6H3Cl3O/c7-3-1-5(9)6(10)2-4(3)8/h1-2,10H
InChI key
LHJGJYXLEPZJPM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2,4,5-Trichlorophenol (2,4,5-TCP) is employed as a raw material in the preparation of various biocides.
Application
2,4,5-trichlorophenol can be used as a starting material to synthesize:
- Hexachlorophene, a fungicide, by the reaction with formaldehyde in the presence of concentrated H2SO4.
- 2,4,5-trichlorophenoxyacetic acid (2,4,5-T), a herbicide.
- N-Benzyl-2-(2,4,5-trichlorophenoxy)acetamide, which is used as an intermediate to prepare 4-benzyl-6,7-dichloro-2H-benzo[b][1,4]oxazin-3(4H)-one via Smiles rearrangement.
The product has been used to study its sorption onto montmorillonite based sorbents.
Disclaimer
“The product is not intended for use as a biocide under global biocide regulations, including but not limited to US EPA′s Federal Insecticide Fungicide and Rodenticide Act, European Biocidal Products Regulation, Canada’s Pest Management Regulatory Agency, Turkey’s Biocidal Products Regulation, Korea’s Consumer Chemical Products and Biocide Safety Management Act (K-BPR) and others."
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
271.4 °F - closed cup
Flash Point(C)
133.0 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Organic Chemistry: With Biological Applications
Organic Chemistry (2011)
Microwave-assisted Synthesis of 2H-Benzo [b][1, 4] oxazin-3 (4H)-ones and 1H-Pyrido [2, 3-b][1, 4] oxazin-2 (3H)-ones via Smiles Rearrangement
Hua Zuo, et al.
Bulletin of the Korean Chemical Society,, 29(7), 1379-1385 (2008)
Photo-assisted degradation of 2, 4, 5-trichlorophenol by Electro-Fe (II)/Oxone{\textregistered} process using a sacrificial iron anode: Performance optimization and reaction mechanism
Wang YR and Chu Wei
Chemical Engineering Journal, 215, 643-650 (2013)
The Lethal Interaction and Formation of a Lipophilic Ternary Complex between 2, 4, 5-Trichlorophenol and the Cu (II)- Bis (1, 10-phenanthroline) Complex
Zhu Ben-Zhan
Chemical Research in Toxicology, 14(2), 222-227 (2001)
Xiaohui Xu et al.
Occupational and environmental medicine, 68(8), 557-561 (2011-05-05)
Trichlorophenols (TCPs) are organochlorine compounds which are ubiquitous in the environment and well known for their carcinogenic effects. However, little is known about their neurotoxicity in humans. Our goal was to examine the association between body burden of TCPs (ie
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service