145491
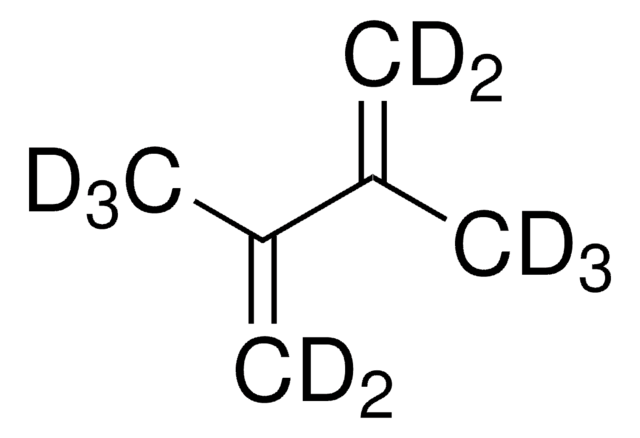
2,3-Dimethyl-1,3-butadiene
98%, contains 100 ppm BHT as stabilizer
Synonym(s):
2,3-Dimethylbuta-1,2-diene, 2,3-Dimethylenebutane, Biisopropenyl, Diisopropenyl
About This Item
Recommended Products
vapor pressure
269 mmHg ( 37.7 °C)
Quality Level
Assay
98%
form
liquid
contains
100 ppm BHT as stabilizer
refractive index
n20/D 1.438 (lit.)
bp
68-69 °C (lit.)
mp
−76 °C (lit.)
density
0.726 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CC(=C)C(C)=C
InChI
1S/C6H10/c1-5(2)6(3)4/h1,3H2,2,4H3
InChI key
SDJHPPZKZZWAKF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
It may be used in the following processes:
- Preparation of 1,3,6-triene derivatives of corresponding 1-aryl-substituted 1,3-dienes by 1,4-hydrobutadienylation in the presence of cobalt catalyst.
- Synthesis of 6-aryl(hetaryl)-3,4-dimethyl-1-nitro-1-cyano-3-cyclohexenes by reacting with gem-cyanonitroethenes.
- As a halogen trap during the study of the photolysis reaction of dibromo adduct of 2,5-diphenyltellurophene.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
30.2 °F - closed cup
Flash Point(C)
-1 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service