144061
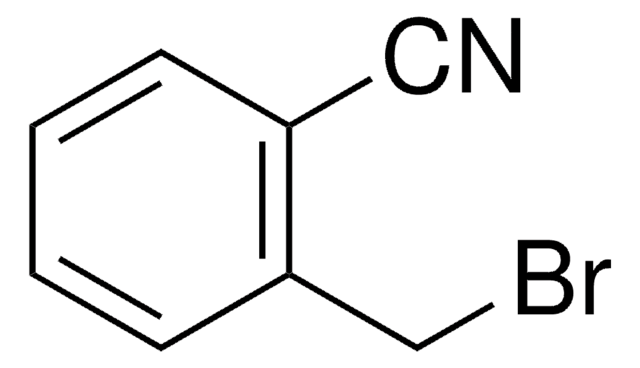
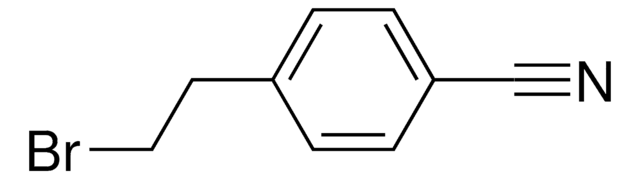
4-(Bromomethyl)benzonitrile
99%
Synonym(s):
α-Bromo-p-tolunitrile, 4-Cyanobenzyl bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrCH2C6H4CN
CAS Number:
Molecular Weight:
196.04
Beilstein:
636717
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
mp
115-117 °C (lit.)
functional group
bromo
SMILES string
BrCc1ccc(cc1)C#N
InChI
1S/C8H6BrN/c9-5-7-1-3-8(6-10)4-2-7/h1-4H,5H2
InChI key
UMLFTCYAQPPZER-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-(Bromomethyl)benzonitrile reacts with 2H-tetrazole in the presence of KOH to yield 4-[(2H-tetra-zol-2-yl)methyl]benzonitrile.
Application
4-(Bromomethyl)benzonitrile may be used in the synthesis of ligands containing a chelating pyrazolyl-pyridine group with a pendant aromatic nitrile.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hazel Fenton et al.
Dalton transactions (Cambridge, England : 2003), 39(16), 3805-3815 (2010-04-08)
Two ligands L(1) and L(2) have been prepared which contain a chelating pyrazolyl-pyridine group with a pendant aromatic nitrile (in L(1), a benzonitrile; in L(2), a naphthonitrile). These ligands react with Ag(I) salts to give a range of infinite coordination
Zheng Xing et al.
Acta crystallographica. Section E, Structure reports online, 64(Pt 2), o445-o445 (2008-01-01)
The title compound, C(9)H(7)N(5), was synthesized by reaction of 4-(bromomethyl)benzonitrile and 2H-tetrazole in the presence of KOH. The relative orientation of the planar tetra-zole ring and the methyl-benzonitrile moiety is (-)-anti-clinal. The crystal packing is dominated by van der Waals
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service