All Photos(1)
About This Item
Linear Formula:
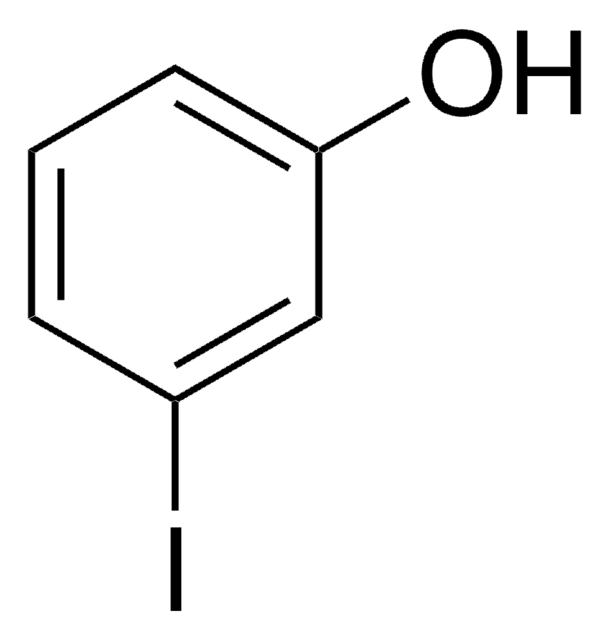
I3C6H2OH
CAS Number:
Molecular Weight:
471.80
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
powder
mp
157-159 °C (lit.)
functional group
iodo
SMILES string
Oc1c(I)cc(I)cc1I
InChI
1S/C6H3I3O/c7-3-1-4(8)6(10)5(9)2-3/h1-2,10H
InChI key
VAPDZNUFNKUROY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,4,6-Triiodophenol is iodinated disinfection byproduct formed during chlorination of sewage effluents. It is a potent thyroid hormone disrupting chemical.
Application
2,4,6-Triiodophenol was used in an in vitro assay to investigate deiodinase activity in human hepatic microsomes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tingting Gong et al.
Chemosphere, 163, 359-365 (2016-08-25)
Iodide is widely present in drinking water sources as well as wastewater effluents. Chlorination and chloramination are the most commonly used disinfection methods. During chlorination or chloramination of drinking water/wastewater effluents, iodide may be oxidized to hypoiodous acid, which may
G J Miroy et al.
Proceedings of the National Academy of Sciences of the United States of America, 93(26), 15051-15056 (1996-12-24)
Transthyretin (TTR) amyloid fibril formation is observed systemically in familial amyloid polyneuropathy and senile systemic amyloidosis and appears to be the causative agent in these diseases. Herein, we demonstrate conclusively that thyroxine (10.8 microM) inhibits TTR fibril formation efficiently in
L García-Capdevila et al.
Journal of chromatography. B, Biomedical sciences and applications, 708(1-2), 169-175 (1998-07-08)
A rapid and simple HPLC method is described for the determination of Bobel-24 (2,4,6-triiodophenol) and other iodinated derivatives in biological samples. The sample preparation was liquid-liquid extraction before injection onto the HPLC system. 2,6-Diiodo-4-methylphenol was used as internal standard. Separation
Matilde Parreño et al.
Molecular cancer therapeutics, 5(5), 1166-1175 (2006-05-30)
2,4,6-Triiodophenol (Bobel-24, AM-24) was originally described as a nonsteroid antiinflammatory molecule. We have synthesized three derivatives of Bobel-24 (Bobel-4, Bobel-16, and Bobel-30) and tested their activities as putative antileukemic agents. We have found that Bobel-24 and Bobel-16 were dual inhibitors
Matilde Parreño et al.
Cancer research, 68(15), 6313-6323 (2008-08-05)
The poor prognosis of pancreatic cancer and poor sensitivity to current therapeutics, associated with resistance to apoptosis, urge the search for new drugs. We previously described the induction of caspase-independent mithochondrial death in leukemia cells by Bobel-24 (AM-24) and derivatives.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service