136964
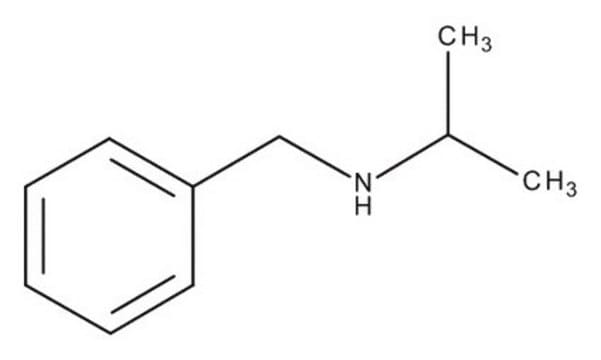
N-Isopropylbenzylamine
97%
Synonym(s):
N-Benzylisopropylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH2NHCH(CH3)2
CAS Number:
Molecular Weight:
149.23
Beilstein:
2638437
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.502 (lit.)
bp
200 °C (lit.)
density
0.892 g/mL at 25 °C (lit.)
functional group
amine
phenyl
SMILES string
CC(C)NCc1ccccc1
InChI
1S/C10H15N/c1-9(2)11-8-10-6-4-3-5-7-10/h3-7,9,11H,8H2,1-2H3
InChI key
LYBKPDDZTNUNNM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
N-Isopropylbenzylamine forms amine adducts with magnesocene at ambient temperature in toluene.
Application
N-Isopropylbenzylamine was used as ligand in the preparation and characterization of bis(cyclopentadienyl)magnesium. It was also used in the synthesis of N-benzylideneisopropylamine-N-oxide.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
161.6 °F - closed cup
Flash Point(C)
72 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
One-flask transformation of secondary amines to nitrones by oxidation with hydrogen peroxide mediated by triscetylpyridinium tetrakis oxodiperoxotungsto-phosphate (PCWP). Some mechanistic considerations.
Ballistreri FP, et al.
Tetrahedron, 48(40), 8677-8684 (1992)
Aibing Xia et al.
Journal of the American Chemical Society, 124(38), 11264-11265 (2002-09-19)
Magnesocene adducts of alkylamines were prepared and characterized. Treatment of 3-amino-2,4-dimethylpentane, isopropylamine, tert-butylamine, benzylamine, or N-isopropylbenzylamine with magnesocene at ambient temperature in toluene afforded the amine adducts Cp2Mg(NH2CH(CH(CH3)2)2) (91%), Cp2Mg(NH2iPr) (80%), Cp2Mg(NH2tBu) (67%), Cp2Mg(NH2CH2Ph) (80%), and Cp2Mg(NH(CH(CH3)2)(CH2C6H5)) (91%). These adducts
O Brüggemann
Biomolecular engineering, 18(1), 1-7 (2001-06-29)
Molecular imprinting is a way of creating polymers bearing artificial receptors. It allows the fabrication of highly selective plastics by polymerizing monomers in the presence of a template. This technique primarily had been developed for the generation of biomimetic materials
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service