134341
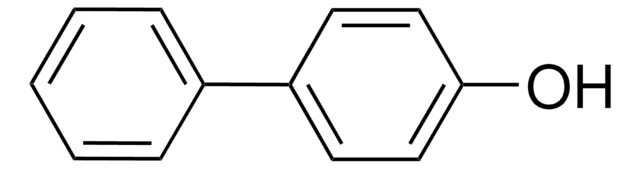
4-Phenylphenol
97%
Synonym(s):
4-Hydroxybiphenyl
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5C6H4OH
CAS Number:
Molecular Weight:
170.21
Beilstein:
1907452
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39023323
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
bp
321 °C (lit.)
mp
164-166 °C (lit.)
solubility
methanol: soluble 50 mg/mL, clear, colorless
functional group
phenyl
SMILES string
Oc1ccc(cc1)-c2ccccc2
InChI
1S/C12H10O/c13-12-8-6-11(7-9-12)10-4-2-1-3-5-10/h1-9,13H
InChI key
YXVFYQXJAXKLAK-UHFFFAOYSA-N
Gene Information
rat ... Ar(24208)
Looking for similar products? Visit Product Comparison Guide
General description
4-Phenylphenol undergoes enzymatic polymerization and polymer developed is characterized by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. It is the intermediate in manufacture of 4-alkyl substituted phenol-formaldehyde resins.
Application
4-Phenylphenol was used in the synthesis of a novel polyphosphazene polyelectrolyte as a dispersing agent of single-walled carbon nanotubes in water.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Skin Irrit. 2 - Skin Sens. 1B
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 2
Flash Point(F)
320.0 °F - closed cup
Flash Point(C)
160 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
MALDI-TOF mass spectrometry characterization of 4-alkyl substituted phenol-formaldehyde novalac type resins.
Mandal H and Hay AS.
Polymer, 38(26), 6267-6271 (1997)
Dispersion of single-walled carbon nanotubes in water with polyphosphazene polyelectrolyte.
Park HJ, et al.
Journal of Inorganic and Organometallic Polymers and Materials, 16(4), 359-364 (2006)
T Yoshimura et al.
Journal of pharmacobio-dynamics, 15(8), 387-393 (1992-08-01)
The apparent in vitro kinetic constants of uridine diphosphate-glucuronyltransferase (UDP-GT) activities towards E6080, 1-naphthol (1-N) and 4-hydroxybiphenyl (4-HB) were determined using microsomes, to assess the effect of inducing agents and evaluate species and tissue differences. In rats, the 3-methylcholanthrene and
C K Kuo et al.
Journal of pharmacobio-dynamics, 14(4), 187-193 (1991-04-01)
Species difference in glucuronidation of morphine was studied using mice, rats, guinea pigs and rabbits in vivo and in vitro. Morphine-3-glucuronide (M-3-G) and morphine-6-glucuronide (M-6-G) were determined by high-performance liquid chromatography. M-3-G was the major urinary metabolite of morphine in
R H Tukey et al.
The Journal of biological chemistry, 268(20), 15260-15266 (1993-07-15)
A polyclonal antibody generated against rabbit liver p-nitrophenol UDP-glucuronosyltransferase (UGT) was used to screen a rabbit liver cDNA expression library constructed in lambda gt11. A 500-base pair cDNA clone, termed pPNP, generated a fusion protein that was antigenic with the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service