All Photos(1)
About This Item
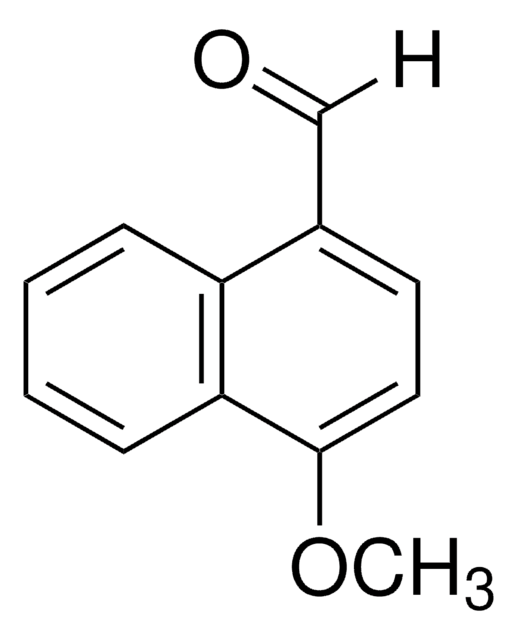
Linear Formula:
HOC10H7CHO
CAS Number:
Molecular Weight:
172.18
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
179-182 °C (lit.)
functional group
aldehyde
SMILES string
Oc1ccc(C=O)c2ccccc12
InChI
1S/C11H8O2/c12-7-8-5-6-11(13)10-4-2-1-3-9(8)10/h1-7,13H
InChI key
LORPDGZOLAPNHP-UHFFFAOYSA-N
Related Categories
Application
4-Hydroxy-1-naphthaldehyde was used in the preparation of racemic 1- and 2-naphthol analogues of tyrosine, 2-amino-3-(4-hydroxy-1-naphthyl)propanoic acid hydrochloride and 2-amino-3-(6-hydroxy-2-naphthyl)propanoic acid hydrobromide.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Zi-Jian Chen et al.
Ecotoxicology and environmental safety, 196, 110533-110533 (2020-04-05)
1-naphthol (1-NAP) is the main metabolite of pesticide carbaryl and naphthalene, and is also a genotoxic and carcinogenic intermediate in the synthesis of organic compound, dyes, pigment and pharmaceutical industry. In this work, two novel haptens were designed and synthesized
Syntheses of 1-and 2-naphthol analogs of DL-tyrosine. Potential fluorescent probes of peptide structure and dynamics in complex environments.
Vela MA, et al.
The Journal of Organic Chemistry, 55(9), 2913-2918 (1990)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service