All Photos(2)
About This Item
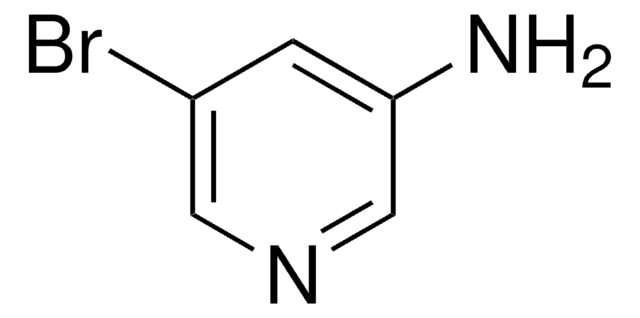
Empirical Formula (Hill Notation):
C5H5BrN2
CAS Number:
Molecular Weight:
173.01
Beilstein:
108737
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
133-138 °C (lit.)
functional group
bromo
SMILES string
Nc1ccc(Br)cn1
InChI
1S/C5H5BrN2/c6-4-1-2-5(7)8-3-4/h1-3H,(H2,7,8)
InChI key
WGOLHUGPTDEKCF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Amino-5-bromopyridine is a brominated aromatic amine reagent and is used for labeling of model reducing-end oligosaccharides via reductive amination.
Application
2-Amino-5-bromopyridine has been used to study the hydrogen-bonding patterns in the 2-amino-5-bromopyridine benzoic acid (1/1) cocrystal. It has been used in the synthesis of 2-amino-5-bromopyridinium 3-aminobenzoate salt and polycyclic azaarenes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tetrahedron Letters, 48, 5039-5039 (2007)
Min Li et al.
Rapid communications in mass spectrometry : RCM, 17(13), 1462-1466 (2003-06-24)
Model reducing-end oligosaccharides were successfully labeled by a brominated aromatic amine reagent, 2-amino-5-bromopyridine (ABP), through reductive amination. Using either a combination of liquid chromatography/electrospray ionization mass spectrometry (LC/ESI-MS) with in-source fragmentation or liquid chromatography/electrospray ionization tandem mass spectrometry (LC/ESI-MS/MS), sequence
Madhukar Hemamalini et al.
Acta crystallographica. Section E, Structure reports online, 66(Pt 3), o664-o664 (2010-01-01)
In the title salt, C(5)H(6)BrN(2) (+)·C(7)H(6)NO(2) (-), the pyridine N atom of the 2-amino-5-bromo-pyridine mol-ecule is protonated. In the crystal, the protonated N atom and the 2-amino group are hydrogen-bonded to the carboxyl-ate O atoms via a pair of N-H⋯O
Madhukar Hemamalini et al.
Acta crystallographica. Section E, Structure reports online, 66(Pt 3), o663-o663 (2010-01-01)
In the title adduct, C(5)H(5)BrN(2)·C(7)H(6)O(2), the carboxyl group of the benzoic acid mol-ecule is twisted away from the attached ring by 12.97 (11)°. The 2-amino-5-bromo-pyridine mol-ecules inter-act with the carboxylic group of neighbouring benzoic acid mol-ecules through N-H⋯O and O-H⋯N hydrogen
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)