122440
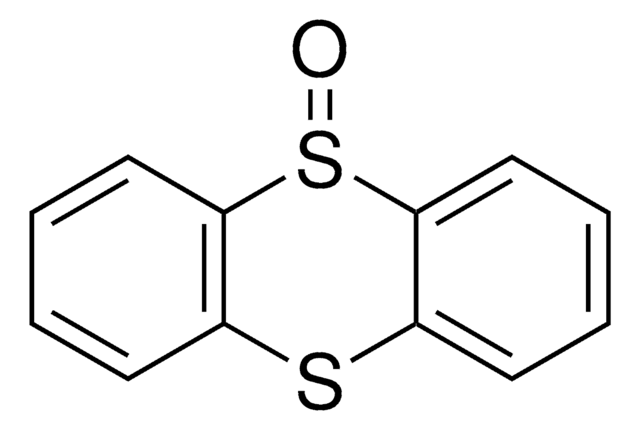
Thianthrene
97%
Synonym(s):
Dibenzodithiodioxane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H8S2
CAS Number:
Molecular Weight:
216.32
Beilstein:
83046
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
bp
364-366 °C (lit.)
mp
151-155 °C (lit.)
solubility
DMF: soluble
H2O: insoluble
SMILES string
S1c2ccccc2Sc3ccccc13
InChI
1S/C12H8S2/c1-2-6-10-9(5-1)13-11-7-3-4-8-12(11)14-10/h1-8H
InChI key
GVIJJXMXTUZIOD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Thianthrene undergoes liquid phase tert-butylation in the presence of large pore zeolites and mesoporous aluminosilicates catalyst to yield tert-butyl derivatives. Thianthrene on oxidation in the presence of hydrogen peroxide and ligninase as catalyst from Phanerochaete chrysosporium yields thianthrene monosulfoxide.
Application
Thianthrene has been used to study aqueous solubilities of several solid nitrogen-, sulfur- and oxygen-containing heterocyclic derivatives of anthracene, phenanthrene and fluorene. It has been used in determination of partition coefficients of several sulfur-containing aromatics in 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide and supercritical carbon dioxide.
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Acid zeolites as catalysts in organic reactions.< i> tert</i>-Butylation of anthracene, naphthalene and thianthrene.
Armengol E, et al.
Applied Catalysis A: General, 149(2), 411-423 (1997)
Pavel Karásek et al.
Journal of chromatography. A, 1140(1-2), 195-204 (2006-12-05)
We report the aqueous solubilities of phenanthrene and several solid three-ring aromatic heterocycles (phenanthridine, acridine, phenazine, thianthrene, phenothiazine, phenoxathiin, phenoxazine, carbazole, dibenzofuran, dibenzothiophene, and 4,6-dimethyldibenzothiophene) at temperatures ranging from 313K to the solute melting point and at a pressure of
R P Schreiner et al.
Applied and environmental microbiology, 54(7), 1858-1860 (1988-07-01)
The oxidation of heterocyclic sulfur compounds reported to be part of the macrostructure of coal and petroleum was investigated. The oxidation of thianthrene solubilized in 10% dimethylformamide to thianthrene monosulfoxide in the presence of hydrogen peroxide was catalyzed by the
Distribution of sulfur-containing aromatics between [hmim][Tf2N] and supercritical CO2: a case study for deep desulfurization of oil refinery streams by extraction with ionic liquids.
Planeta J, et al.
Green Chemistry, 8(1), 70-77 (2006)
Patrick Frank et al.
Inorganic chemistry, 45(24), 9864-9876 (2006-11-23)
Sulfur K-edge X-ray absorption spectroscopy (XAS) was used to characterize the approximately 0.1% sulfur found both in native reticulated vitreous carbon (RVC) foam and in RVC oxidatively modified using 0.2 M KMnO4 in 2 M H2SO4. Sulfur valences and functional
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service