122351
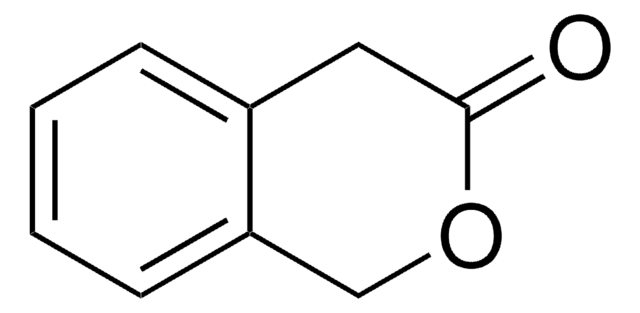
4-Chromanone
97%
Synonym(s):
2,3-Dihydro-1-benzopyran-4-one
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H8O2
CAS Number:
Molecular Weight:
148.16
Beilstein:
124652
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
refractive index
n20/D 1.575 (lit.)
bp
127-128 °C/13 mmHg (lit.)
mp
35-38 °C (lit.)
SMILES string
O=C1CCOc2ccccc12
InChI
1S/C9H8O2/c10-8-5-6-11-9-4-2-1-3-7(8)9/h1-4H,5-6H2
InChI key
MSTDXOZUKAQDRL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-Chromanone has been used to study the substrate specificity and catalytic ability of 4-hydroxyacetophenone monooxygenase isolated from Pseudomonas fluorescens ACB.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Zahra Najafi et al.
Bioorganic chemistry, 83, 303-316 (2018-11-06)
A new series of tacrine-coumarin hybrids linked to 1,2,3-triazole were designed, synthesized, and tested as potent dual binding site cholinesterase inhibitors (ChEIs) for the treatment of Alzheimer's disease (AD). Among them, compound 8e was the most potent anti-AChE derivative (IC50 = 27 nM)
Gyeong-Je Lee et al.
Biological & pharmaceutical bulletin, 38(8), 1199-1207 (2015-08-04)
The aim of this study was to examine the anabolic and anticatabolic functions of bavachin in primary rat chondrocytes. With bavachin treatment, chondrocytes survived for 21 d without cell proliferation, and the proteoglycan content and extracellular matrix increased. Short-term monolayer culture
U Thull et al.
Biochemical pharmacology, 47(12), 2307-2310 (1994-06-15)
A number of unsubstituted aromatic hydrocarbons, azaheterocycles, oxaheterocycles and cyclic ketones were screened for their inhibitory potency towards monoamine oxidases (MAO; EC 1.4.3.4.) A and B. Fair activities (IC50 10-100 microM) and selectivities were found for, e.g. naphthalene, anthracene, phenanthrene
A R Ibrahim et al.
Journal of natural products, 53(6), 1471-1478 (1990-11-01)
Microbial transformation of chromone, chromanone, and 3 ring A hydroxyflavones (5-hydroxy-, 6-hydroxy-, and 7-hydroxyflavones) was attempted using thirty-two microorganisms. While chromone was not biotransformed, chromanone was transformed to chromone and chromanol by Aspergillus niger in 2% yield. Ring A hydroxylated
G R Harlow et al.
The Journal of biological chemistry, 272(9), 5396-5402 (1997-02-28)
Alanine-scanning mutagenesis was performed on amino acid residues 210-216 of cytochrome P450 3A4, the major drug-metabolizing enzyme of human liver. Mutagenesis of this region, which has been proposed to align with the C-terminal ends of F-helices from cytochromes P450BM-3, P450terp
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service