116238
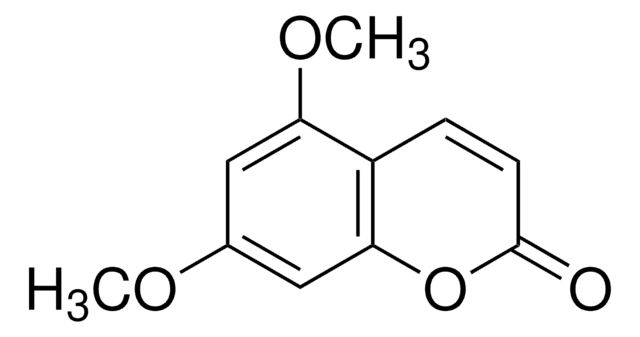
5,7-Dimethoxycoumarin
98%
Synonym(s):
Citropten, Limettin
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C11H10O4
CAS Number:
Molecular Weight:
206.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
146-149 °C (lit.)
functional group
ester
SMILES string
COc1cc(OC)c2C=CC(=O)Oc2c1
InChI
1S/C11H10O4/c1-13-7-5-9(14-2)8-3-4-11(12)15-10(8)6-7/h3-6H,1-2H3
InChI key
NXJCRELRQHZBQA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
5,7-dimethoxycoumarin is isolated and identified from leaves and fruits of Pelea anisata H. Mann, a plant whose fruit are used in the construction of mohikana leis. It induces frameshift mutagenesis in bacteria. It also causes lethal photosensitization and the formation of sister chromatid exchanges in Chinese hamster cells.
Biochem/physiol Actions
5,7-Dimethoxycoumarin induces the processes of differentiation and melanogenesis in murine (B16) and human (A375).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jinglan Han et al.
Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica, 34(17), 2200-2202 (2009-12-01)
To study the chemical constituents from Lobelia chinensis. The constituents were extracted with 95% EtOH, partitioned with different solvents, and isolated and purified by silica gel column chromatography and crystallization, their structures were elucidated by physico-chemical properties and spectroscopic data.
E Gorgus et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 48(1), 93-98 (2009-09-23)
Phototoxic and photo-genotoxic furocoumarins occur, e.g., in citrus species, parsnip, parsley, celery, and figs. They exhibit phototoxic and photo-genotoxic properties in combination with UV radiation, while less is known about the phototoxicity of the coumarin derivative limettin mainly found in
Daniela Alesiani et al.
International journal of oncology, 32(2), 425-434 (2008-01-19)
In the present study we investigated the antiproliferative activity of 5,7-dimethoxycoumarin on the murine B16 and human A375 melanoma cell lines. The inhibitory concentration 50 (IC50) was estimated for each cell line by preliminary assay of tetrazolium salt reduction (MTT).
Earl Grey tea intoxication.
Josef Finsterer
Lancet (London, England), 359(9316), 1484-1484 (2002-05-04)
D Chouchi et al.
Journal of chromatography. A, 672(1-2), 177-183 (1994-06-24)
Generally on the gas chromatogram of a volatile essential oil, terpenes, oxygenated compounds and sesquiterpenes appear. With temperature programming, it was shown that some non-volatiles are present with the volatiles. They are simple coumarin (2H-1-benzopyran-2-one) derivatives such as citropten (5,7-dimethoxycoumarin)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service