114693
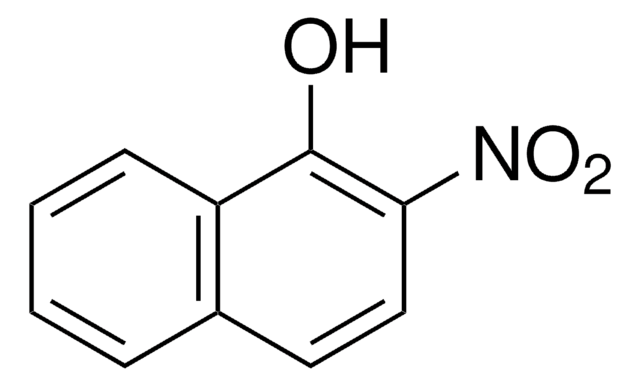
1-Nitroso-2-naphthol
97%
Synonym(s):
1-Nitroso-2-hydroxynaphthalene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ONC10H6OH
CAS Number:
Molecular Weight:
173.17
Colour Index Number:
10005
Beilstein:
776947
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
106-108 °C (dec.) (lit.)
functional group
C-nitroso
nitroso
SMILES string
Oc1ccc2ccccc2c1N=O
InChI
1S/C10H7NO2/c12-9-6-5-7-3-1-2-4-8(7)10(9)11-13/h1-6,12H
InChI key
YXAOOTNFFAQIPZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1-Nitroso-2-naphthol is the chelating ion-exchanger in the synthesis of alumina adsorbents that are of acidic, basic and neutral nature. It is used for the removal and preconcentration of Pb(II), Cu(II), Cr(III) from waste, as well as drinking water. It is also used in the preconcentration of cobalt using C(18) microcolumn with flow injection that is coupled to a flame atomic absorption spectrometry (FI-FAAS) system.
1-Nitroso-2-naphthol is used in the preparation of 1-nitroso-2-naphthol-sodium nitrite reagent.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Muta. 2 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
[A modified method of determination of bound 5-hydroxyindoleacetic acid in the urine].
N G Shafranova et al.
Laboratornoe delo, (7)(7), 25-26 (1983-01-01)
N-acetyl-L-cysteine: an alternative to 2-mercaptoethanol in the reduction of Udenfriend's chromophore used for the determination of urinary 5-hydroxyindoleacetic acid.
M Zouheir Habbal
Clinica chimica acta; international journal of clinical chemistry, 130(2), 251-256 (1983-05-30)
C F Burrows et al.
Journal of the American Veterinary Medical Association, 183(3), 318-322 (1983-08-01)
The nitrosonapthol test, which qualitatively measures urinary excretion of 4-hydroxyphenylacetic acid and related compounds, was evaluated as a screening test for small intestinal disease in 60 dogs with chronic diarrhea. Test results were positive in 8 of 13 dogs with
Nayyef Aljaar et al.
The Journal of organic chemistry, 78(1), 154-166 (2012-11-28)
Reactions between 1-nitroso-2-naphthols and α-functionalized ketones such as α-bromo-, α-chloro-, α-mesyloxy-, α-tosyloxy-, and α-hydroxy ketones under basic conditions delivered 2-substituted naphtho[1,2-d][1,3]oxazoles in a single synthetic operation. The product formation was accompanied by the unexpected loss of the C═O group from
J A Knight et al.
Clinical chemistry, 29(11), 1969-1971 (1983-11-01)
1-Nitroso-2-naphthol reacts with various 5-hydroxyindoles as well as para-substituted phenols and guaiacols. Consequently, this reaction has long been used to measure tyrosine in tyrosinosis and tyrosinemia, homovanillic acid in neuroblastoma, and 5-hydroxy-3-indoleacetic acid in the carcinoid tumor. We report here
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service