112747
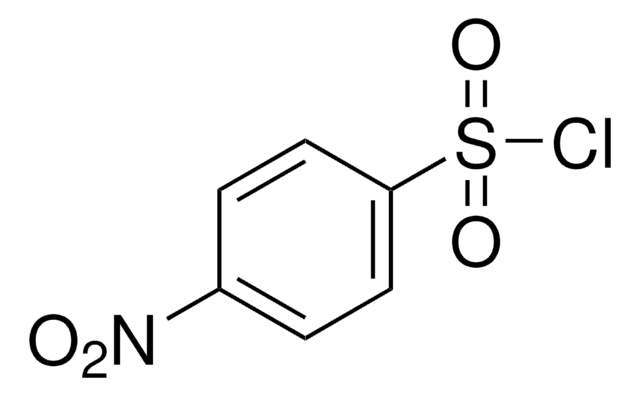
N-Acetylsulfanilyl chloride
98%
Synonym(s):
4-Acetamidobenzenesulfonyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
4-(CH3CONH)C6H4SO2Cl
CAS Number:
Molecular Weight:
233.67
Beilstein:
746676
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
142-145 °C (dec.) (lit.)
functional group
amide
SMILES string
CC(=O)Nc1ccc(cc1)S(Cl)(=O)=O
InChI
1S/C8H8ClNO3S/c1-6(11)10-7-2-4-8(5-3-7)14(9,12)13/h2-5H,1H3,(H,10,11)
InChI key
GRDXCFKBQWDAJH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
L-hydroxyproline has been derivatized with N-acetylsulfanilyl chloride and 5-chlorovaleric acid during the synthesis of the haptens HP1 and HP2.
Application
N-Acetylsulfanilyl chloride is used in the synthesis of N1,N1-dimethylsulfanilamide as the internal standard in the reaction. The reaction is used to determine tramadol and its O-desmethylated metabolite in blood plasma.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Li L Wang et al.
Journal of environmental science and health. Part. B, Pesticides, food contaminants, and agricultural wastes, 48(1), 9-15 (2012-10-04)
China's government has prohibited the addition of simply hydrolyzed animal protein from solid leather waste into milk. The objective of this study was to produce a monoclonal antibody against L-hydroxyproline, a special amino acid in hydrolyzed animal protein. L-hydroxyproline was
Ning Li et al.
European journal of medicinal chemistry, 155, 531-544 (2018-06-18)
Ten novel symmetric 3,5-bis(arylidene)-4-piperidone derivatives (BAPs, 1-10) and fourteen dissymmetric BAPs (11-24) were synthesized and evaluated the cytotoxicity. All of the compounds have been screened for their anti-inflammatory activity characterized by evaluating their inhibitory effects on LPS-induced IL-6, TNF-α secretion.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service