All Photos(2)
About This Item
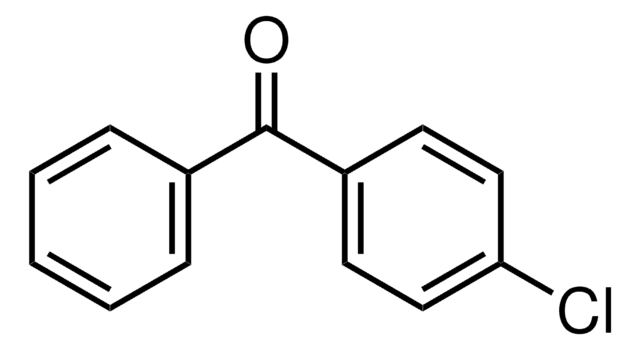
Linear Formula:
H2NC6H3(Cl)COC6H4Cl
CAS Number:
Molecular Weight:
266.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
mp
87-89 °C (lit.)
solubility
methanol: soluble
functional group
chloro
ketone
SMILES string
Nc1ccc(Cl)cc1C(=O)c2ccccc2Cl
InChI
1S/C13H9Cl2NO/c14-8-5-6-12(16)10(7-8)13(17)9-3-1-2-4-11(9)15/h1-7H,16H2
InChI key
KWZYIAJRFJVQDO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Amino-2′,5-dichlorobenzophenone can be synthesized from a precursor diazepine, Iorazepam.
Application
2-Amino-2′,5-dichlorobenzophenone was used as electrophilic coupling spectrophotometric reagent to develop a new, fast and accurate spectrophotometric method for the determination of hexavalent chromium in environmental samples. 2-Amino-2′,5-dichlorobenzophenone was used to study electroanalytical behaviour of mexazolam.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
F Guan et al.
Journal of analytical toxicology, 23(1), 54-61 (1999-02-18)
Benzodiazepines are common drugs that cause intoxication. Benzodiazepines and their metabolites can be converted by hydrolysis in acid to the corresponding benzophenones, which are easier to be separated from matrices because of their hydrophobic properties. In this study, a new
Electroanalytical study of a benzodiazepinooxazole: Mexazolam.
Iturbe A, et al.
Fresenius Journal of Analytical Chemistry, 345(6), 451-455 (1993)
Exploiting of the phenoxazine as first-ever use ligand in rapid spectrophotometric methods for the determination of chromium (VI) in environmental samples.
Galil MSA and Ra AO.
Analele Universitatii Ovidius din Constanta-Seria Chimie, 23(2), 180-186 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service