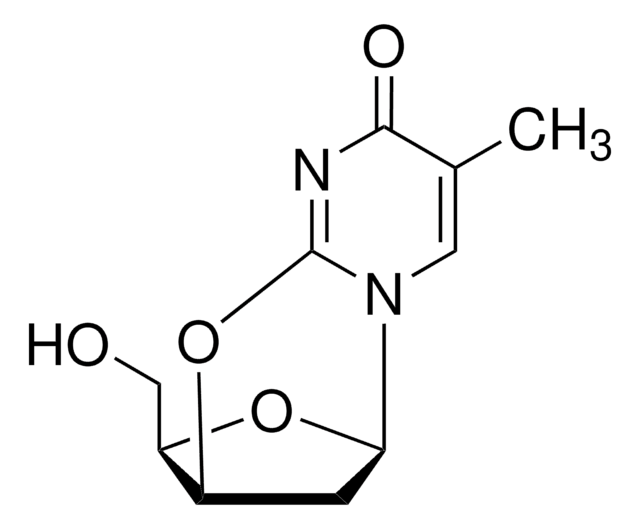
A6007
Apotryptophanase from Escherichia coli
soluble powder, 75-150 units/mg solid
Synonym(s):
Tryptophanase from Escherichia coli, L-Tryptophan indole-lyase (deaminating)
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
biological source
Escherichia coli
Quality Level
form
soluble powder
specific activity
75-150 units/mg solid
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Application
Apotryptophanase is used for the quantitative determination of pyridoxal-phosphate. Apotryptophanase, from Sigma, has been used to study pregnancy-associated PLP deficiency and vitamin B-6 deficiency .
Biochem/physiol Actions
Apotryptophanase hydrolizes tryptophan and is capable of catalyzing α,β-elimination reactions with a number of substituted amino acids, including S-methyl-, S-ethyl- and S-benzyl- L-cysteine. DTNB inactivates tryptophanase .
Unit Definition
One unit releases one μg of indole from L-tryptophan in 10 min at pH 8.3 at 37 °C in the presence of 0.04 mM pyridoxal-5′--phosphate.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Takako Sasaki-Imamura et al.
Applied and environmental microbiology, 76(13), 4260-4268 (2010-05-18)
The l-tryptophan degradation product indole is a purported extracellular signaling molecule that influences biofilm formation in various bacteria. Here we analyzed the mechanisms of indole production in Fusobacterium nucleatum and the effects of tryptophan and indole on F. nucleatum planktonic
Nicholas S Wigginton et al.
Environmental science & technology, 44(6), 2163-2168 (2010-02-18)
Here we describe results from a proteomic study of protein-nanoparticle interactions to further the understanding of the ecotoxicological impact of silver nanoparticles (AgNPs) in the environment. We identified a number of proteins from Escherichia coli that bind specifically to bare
Anna Kogan et al.
BMC structural biology, 9, 65-65 (2009-10-10)
Oligomeric enzymes can undergo a reversible loss of activity at low temperatures. One such enzyme is tryptophanase (Trpase) from Escherichia coli. Trpase is a pyridoxal phosphate (PLP)-dependent tetrameric enzyme with a Mw of 210 kD. PLP is covalently bound through
Birgit Seidelt et al.
Science (New York, N.Y.), 326(5958), 1412-1415 (2009-11-26)
Expression of the Escherichia coli tryptophanase operon depends on ribosome stalling during translation of the upstream TnaC leader peptide, a process for which interactions between the TnaC nascent chain and the ribosomal exit tunnel are critical. We determined a 5.8
Elzbieta Winnicka et al.
Isotopes in environmental and health studies, 46(2), 225-232 (2010-06-29)
The kinetic and solvent deuterium isotope effects in the 4- and 5-positions of the indole ring on the enzymatic decomposition of l-tryptophan catalysed by the enzyme TPase (EC. 4.1.99.1) were determined. The isotope effects were investigated by the non-competitive method
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service