696307
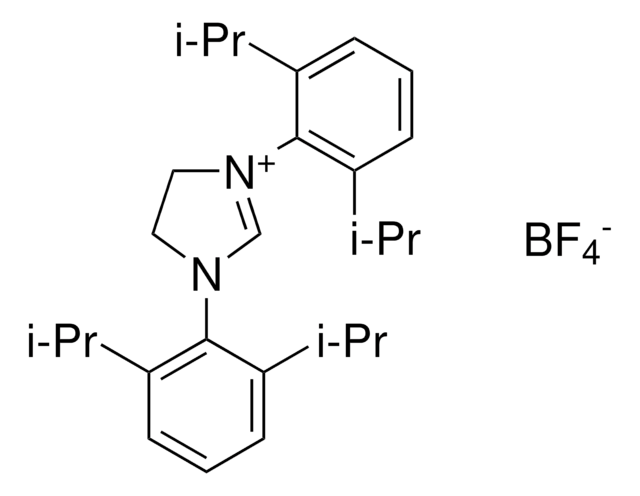
Chloro[1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene]copper(I)
Synonym(s):
[(iPr)CuCl], [1,3-Bis(2,6-diisopropylphenyl)imidazol-2-ylidene]copper(I) chloride
About This Item
Recommended Products
form
powder
reaction suitability
core: copper
reagent type: catalyst
mp
>300 °C
SMILES string
CC(C)c1cccc(C(C)C)c1N2C=CN(\C2=[Cu]\Cl)c3c(cccc3C(C)C)C(C)C
InChI
1S/C27H36N2.ClH.Cu/c1-18(2)22-11-9-12-23(19(3)4)26(22)28-15-16-29(17-28)27-24(20(5)6)13-10-14-25(27)21(7)8;;/h9-16,18-21H,1-8H3;1H;/q;;+1/p-1
InChI key
JPUFNIIPFXQOCB-UHFFFAOYSA-M
Application
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
N-Heterocyclic Carbene-Copper Complexes
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![[(IPr)AuCl] Umicore](/deepweb/assets/sigmaaldrich/product/structures/186/572/1f89dfca-fb52-46a2-9c9d-96db67c22883/640/1f89dfca-fb52-46a2-9c9d-96db67c22883.png)
![Chloro[1,3-Bis(2,4,6-trimethylphenyl)imidazol-2-ylidene]copper(I) 95%](/deepweb/assets/sigmaaldrich/product/structures/160/888/97509eeb-0719-4853-aaae-8a9d02f4f7ad/640/97509eeb-0719-4853-aaae-8a9d02f4f7ad.png)

![[1,3-Bis(2,6-diisopropylphenyl)imidazol-2-ylidene] [bis(trifluoromethanesulfonyl)imide]gold(I) 95%](/deepweb/assets/sigmaaldrich/product/structures/336/250/c96c15b3-1a1c-479f-a588-76e98905be23/640/c96c15b3-1a1c-479f-a588-76e98905be23.png)



![2-Mesityl-5-methylimidazo[1,5-a]pyridinium chloride 97%](/deepweb/assets/sigmaaldrich/product/structures/495/055/5d86d2cc-b538-4586-9e2c-9e0d870826a7/640/5d86d2cc-b538-4586-9e2c-9e0d870826a7.png)