All Photos(2)
About This Item
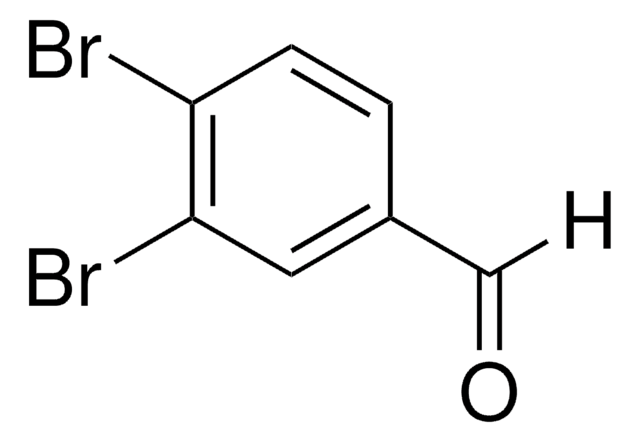
Linear Formula:
Br2C6H3CHO
CAS Number:
Molecular Weight:
263.91
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
mp
84-88 °C (lit.)
SMILES string
Brc1cc(Br)cc(C=O)c1
InChI
1S/C7H4Br2O/c8-6-1-5(4-10)2-7(9)3-6/h1-4H
InChI key
ZLDMZIXUGCGKMB-UHFFFAOYSA-N
Related Categories
Application
Reactant involved in:
- Suzuki-Miyaura cross-coupling reactions
- Synthesis of blue fluorescent dye derivatives for organic light emitting diodes
- Sharpless kinetic resolution for the formation of Baylis-Hillman enal adducts
- Synthesis of podophyllotoxin mimetic pyridopyrazoles as anticancer agents
- Allylic alkylation
- Synthesis of C2-symmetric biphosphine ligand I
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jakub Saadi et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 19(12), 3842-3845 (2013-02-21)
Double-action haloketones: A super silyl group enabled the first highly diastereoselective Mukaiyama aldol reactions of α-chloro- and α-fluoroketones with a wide range of aldehydes, providing anti-β-siloxy-α-haloketones. This process is compatible with one-pot double-aldol methodology and allows for rapid access to
Punit P Seth et al.
Bioorganic & medicinal chemistry letters, 14(22), 5569-5572 (2004-10-16)
The preparation and evaluation of novel aryl urea analogs as broad-spectrum antibacterial agents is described. Numerous compounds showed low micromolar minimum inhibitory concentrations (MIC) against both Gram-positive and Gram-negative bacteria. Selected analogs also exhibited in vivo efficacy in a lethal
Synthesis and luminescence characteristics of conjugated dendrimers with 2, 4, 6-triaryl-1, 3, 5-triazine periphery.
Kim CK, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 44(1), 254-263 (2006)
Coordination-Driven Self-Assembly of Fullerene-Functionalized Pt (II) Metallacycles.
Neti VSPK, et al.
Organometallics, 34(20), 4813-4815 (2015)
Conformational Behavior of Conjugated Polymers With Oligo (phenylene vinylene) Side Chains.
Peeter H and Koeckelberghs G.
Macromolecular Chemistry and Physics, 214(5), 538-546 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service