SRP6310
Calmodulin from bovine brain
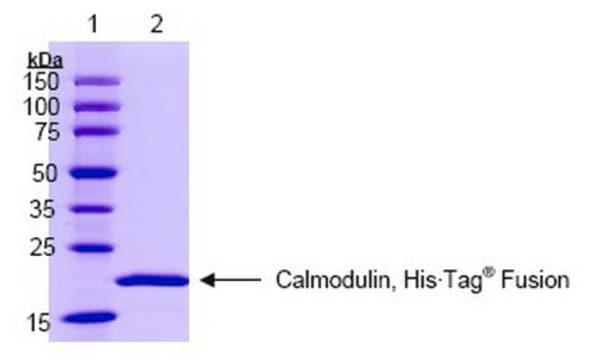
≥95% (SDS-PAGE)
Synonym(s):
CALM, CAM
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352200
NACRES:
NA.32
Recommended Products
biological source
bovine brain
Assay
≥95% (SDS-PAGE)
form
lyophilized
mol wt
16 kDa
packaging
pkg of 1 mg
pkg of 500 μg
suitability
suitable for chromatography
UniProt accession no.
shipped in
wet ice
storage temp.
−20°C
Gene Information
bovine ... CAM(520277)
General description
Calmodulin (CaM) is a ubiquitous, calcium-binding protein. CaM is expressed in many cell types and can have different subcellular locations, including the cytoplasm, within organelles, or associated with the plasma or organelle membranes. Many of the proteins that CaM binds are unable to bind calcium themselves, and as such use CaM as a calcium sensor and signal transducer. CaM can also make use of the calcium stores in the endoplasmic reticulum, and the sarcoplasmic reticulum. CaM undergoes a conformational change upon binding to calcium, which enables it to bind to specific proteins for a specific response.
Application
Calmodulin from bovine brain has been used to study calmodulin-associated endothelium-derived relaxing factor/nitric oxide synthase activity in the particulate and cytosolic fractions of bovine aortic endothelial cells. It has also been used as a standard in size-exclusion chromatography.
Biochem/physiol Actions
Calmodulin (CaM) can bind to and regulate a multitude of different protein targets, thereby affecting many different cellular functions. It is involved in inflammation, metabolism, apoptosis, muscle contraction, intracellular movement, short-term and long-term memory, nerve growth and the immune response. CaM can bind up to four calcium ions, and can undergo post-translational modifications, such as phosphorylation, acetylation, methylation and proteolytic cleavage, each of which can potentially modulate its actions.
Physical form
Lyophilized in 30 mM Hepes, pH 7.4, 1 mM CaCl2 and 0.1 mM DTT.
Reconstitution
In water or aqueous buffer
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Calmodulin methyltransferase is an evolutionarily conserved enzyme that trimethylates Lys-115 in calmodulin.
Magnani R, et al.
Nature Communications, 43, doi: 10-doi: 10 (2010)
Ca2+ binding and conformational change in two series of point mutations to the individual Ca(2+)-binding sites of calmodulin.
Maune JF, et al.
The Journal of Biological Chemistry, 267, 5286-5295 (1992)
Calmodulin-dependent endothelium-derived relaxing factor/nitric oxide synthase activity is present in the particulate and cytosolic fractions of bovine aortic endothelial cells.
Forstermann U, et al.
Proceedings of the National Academy of Sciences of the USA, 88, 1788-1792 (1991)
Calmodulin is a subunit of nitric oxide synthase from macrophages.
Cho HJ, et al.
The Journal of Experimental Medicine, 176, 599-604 (1992)
Intestinal calmodulin and calcium-binding protein differ in their distribution and in the effect of vitamin D steroids on their concentration.
Thomasset M, et al.
Febs Letters, 127, 13-16 (1981)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service