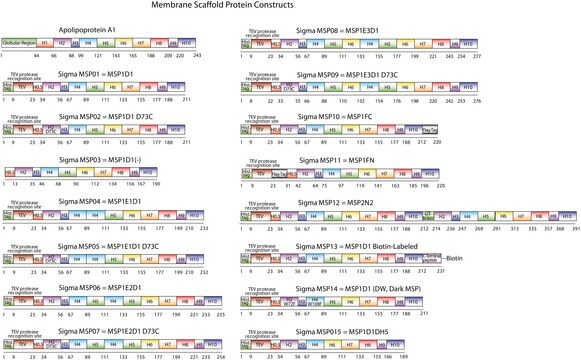
MSP09
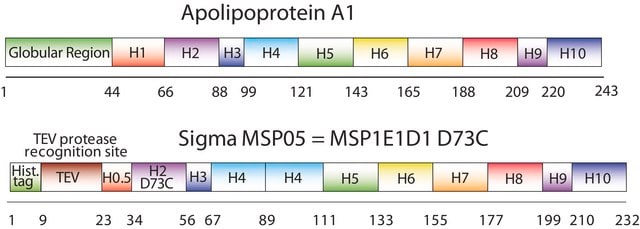
Membrane Scaffold Protein 1E3D1 D73C
recombinant, expressed in E. coli, Cysteine substituted at position 73
About This Item
Recommended Products
recombinant
expressed in E. coli
Assay
≥90% (SDS-GE)
form
buffered aqueous solution
shipped in
ambient
storage temp.
−20°C
General description
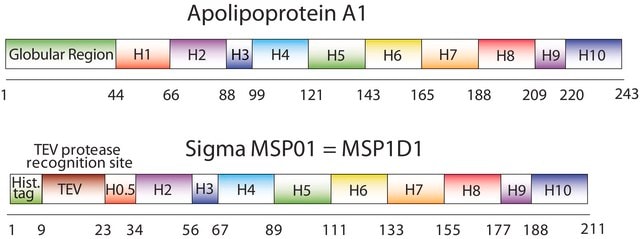
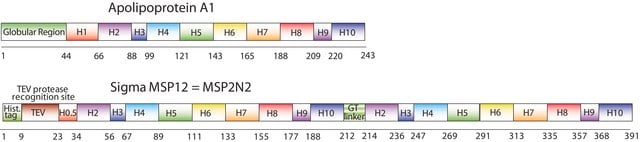
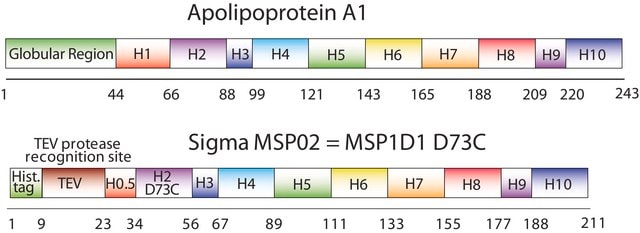
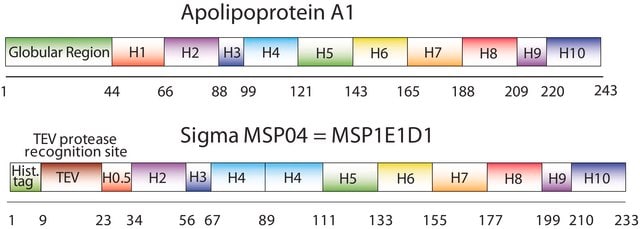
The first MSP, MSP1, was engineered with its sequence based on the sequence of A-1, but without the globular N-terminal domain of native A-1. The MSP1E3D1 D73C variant of MSP1 differs from MSP1 in the following facets:
- It deletes the first 11 amino acids in the Helix 1 portion (referred to as “H0.5” in the accompanying figure) of the original MSP1 sequence3 (which is known separately as MSP1D1).
- It repeats the Helix 4 (H4), Helix 5 (H5) and Helix 6 (H6) sequences of the original MSP1 sequence between the parent Helix 6 (H6) and Helix 7 (H7) segments of MSP1D1.
- It substitutes a cysteine (C) residue for an aspartic acid (D) residue in the Helix 2 (H2) portion of the protein, at position 73 of the original native A-1 sequence.
- The initial histidine-tag is one amino acid shorter.
Application
Legal Information
- 7,691,414 Membrane scaffold proteins
- 7,662,410 Membrane scaffold proteins and embedded membrane proteins
- 7,622,437 Tissue factor compositions and methods
- 7,592,008 Membrane scaffold proteins
- 7,575,763 Membrane scaffold proteins and tethered membrane proteins
- 7,083,958 Membrane scaffold proteins
- 7,048,949 Membrane scaffold proteins
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
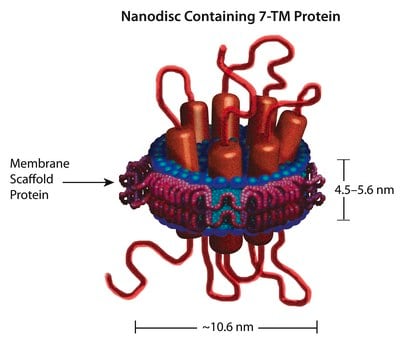
Nanodisc technology aids membrane protein solubilization, overcoming associated challenges in diverse protein classes.
Nanodisc technology aids membrane protein solubilization, overcoming associated challenges in diverse protein classes.
Nanodisc technology aids membrane protein solubilization, overcoming associated challenges in diverse protein classes.
Nanodisc technology aids membrane protein solubilization, overcoming associated challenges in diverse protein classes.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service