58689
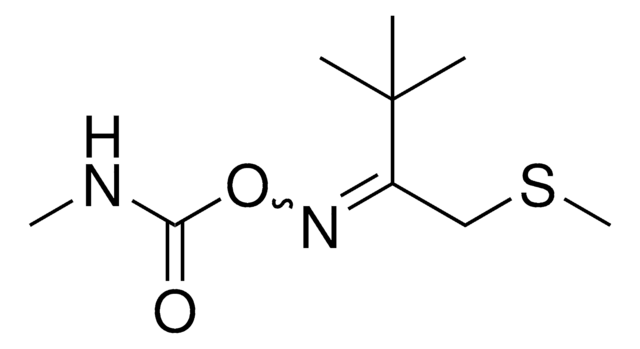
N-Isobutyryl-D-cysteine
for chiral derivatization, LiChropur™, ≥97.0%
Synonym(s):
N-(2-Methylpropionyl)-D-cysteine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HSCH2CH[NHCOCH(CH3)2]CO2H
CAS Number:
Molecular Weight:
191.25
MDL number:
UNSPSC Code:
12000000
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
for chiral derivatization
Quality Level
product line
ChiraSelect™
Assay
≥97.0% (RT)
≥97.0%
form
solid
optical purity
enantiomeric ratio: ≥99.5:0.5 (HPLC)
quality
LiChropur™
mp
97-101 °C (lit.)
97-101 °C
storage temp.
2-8°C
SMILES string
CC(C)C(=O)N[C@H](CS)C(O)=O
InChI
1S/C7H13NO3S/c1-4(2)6(9)8-5(3-12)7(10)11/h4-5,12H,3H2,1-2H3,(H,8,9)(H,10,11)/t5-/m1/s1
InChI key
BWBQXMAXLAHHTK-RXMQYKEDSA-N
Looking for similar products? Visit Product Comparison Guide
General description
It was used for derivatization of amino acid mixtures of OPA during HPLC analysis of L- and D-amino acids in plants.
N-Isobutyryl-D-cysteine is a chiral thiol mostly used in precolumn orthophthaldehyde (OPA) derivatization of amino acids.
Legal Information
ChiraSelect is a trademark of Sigma-Aldrich Co. LLC
LiChropur is a trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Chromatographic determination of L- and D-amino acids in plants.
Bruckner H and Westhauser T.
Amino Acids, 24 (1-2), 43-55 (2003)
Handbook of HPLC
Danilo Corradini
Science, 715-715 (2011)
Jan Bergmann et al.
Analytical and bioanalytical chemistry, 378(6), 1624-1629 (2004-06-25)
A fast and sensitive method was developed for the determination of the absolute configuration of selenomethionine. The enantiomers of selenomethionine were converted into diastereomeric isoindole derivatives by reaction with o-phthaldialdehyde and N-isobutyryl-L-cysteine. This easy-to-handle reaction proceeds quantitatively in a few
Haiqing Liang et al.
Physical chemistry chemical physics : PCCP, 12(17), 4431-4434 (2010-04-22)
All-atomistic molecular dynamics simulations with explicit water solution are performed to investigate the interaction between single-stranded DNA (ssDNA) molecules and chiral N-isobutyryl-cysteine (NIBC) molecule coated Au surfaces. Different contributions to the force exerted on ssDNA are analyzed. It turns out
A Ekberg-Jansson et al.
The European respiratory journal, 13(4), 829-834 (1999-06-11)
N-isobutyrylcysteine (NIC), a new thiol compound that is not rapidly hydrolysed to give higher levels of free thiols in the body than N-acetylcysteine (NAC), was used to test if the effect of NAC on exacerbations in chronic bronchitis was an
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service