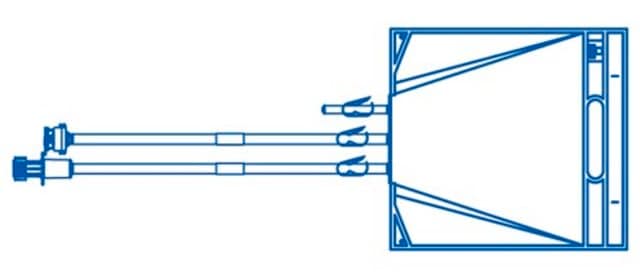
FRZ0001L001P5
Mobius® Gold 2D Freeze Assembly w/Helium IT
1L PureFlex™ film with AseptiQuik® S connectors
Synonym(s):
Mobius®, Gold w/Helium IT, 1L PF FRZ TRN Assy w/ ASPQ S
About This Item
Recommended Products
material
PureFlex™ device (film)
connections (AseptiQuik S connector)
tubing (Pharma 65)
Quality Level
Agency
certified by the (Assembly performed under ISO Clean Room Class 7 conditions in a facility)
certified by the ISO (Bags manufactured under ISO Clean Room Class 8 conditions in a facility)
sterility
sterile; γ-irradiated (level of 25-40 kGY)
product line
Mobius® Freeze
packaging
package of 5 ×
parameter
-80-60 °C temp. range (112-140 °F)
1 L process volume
Looking for similar products? Visit Product Comparison Guide
General description
Application
Packaging
Components
Other Notes
Legal Information
Not finding the right product?
Try our Product Selector Tool.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
This article explains how to streamline transfer of an allogeneic cell therapies process to a CDMO partner and long-term manufacturing success.
This article explains how to streamline transfer of an allogeneic cell therapies process to a CDMO partner and long-term manufacturing success.
This article explains how to streamline transfer of an allogeneic cell therapies process to a CDMO partner and long-term manufacturing success.
This article explains how to streamline transfer of an allogeneic cell therapies process to a CDMO partner and long-term manufacturing success.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service