208713
Calpain-1, Human Erythrocytes
Calpain-1, Human Erythrocytes, is a native calpain-1. A heterodimeric cysteine proteinase with low Ca2+ requirement (EC₅₀ = 2 µM).
Synonym(s):
μ-Calpain
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
General description
Native calpain-1 from human erythrocytes. Ca2+-dependent cysteine proteinase with low Ca2+ requirement (half-maximal activation = 2 µM). Participates in the ATP release reaction of platelets stimulated with thrombin.
Native calpain-1 from human erythrocytes. Ca2+-dependent heterodimeric cysteine proteinase with low Ca2+ requirement (EC50= 2 µM).
Packaging
Please refer to vial label for lot-specific concentration.
Warning
Toxicity: Harmful (C)
Unit Definition
One unit is defined as the amount of enzyme that will hydrolyze 1 pmol Suc-LLVY-AMC in 1 min, 25°C using the Calpain Activity Assay Kit, Fluorogenic (Cat. No. QIA120). Note: 1 caseinolytic unit = 1 fluorogenic unit.
Physical form
In 20 mM imidazole, 5 mM β-mercaptoethanol, 1 mM EDTA, 1 mM EGTA, 30% glycerol, pH 6.8.
Preparation Note
Prepared from blood that has been shown by certified tests to be negative for HBsAg and for antibodies to HIV and HCV.
Reconstitution
Following initial thaw, aliquot and freeze (-70°C).
Analysis Note
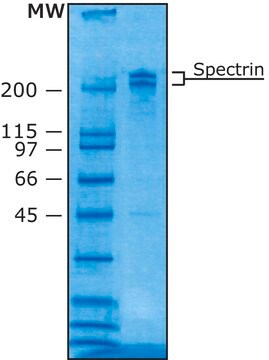
Comparable to reference lot by SDS-PAGE
Other Notes
Vanderklish, P.W., and Bahr, B.A. 2000. Int. J. Exp. Pathol.81, 323.
Sorimachi, H., et al. 1997. Biochem. J. 328, 721.
Croall, D.E., and McGrody, K.S. 1994. Biochemistry33, 13223.
Sorimachi, H., et al. 1997. Biochem. J. 328, 721.
Croall, D.E., and McGrody, K.S. 1994. Biochemistry33, 13223.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Peter Tompa et al.
The Journal of biological chemistry, 277(11), 9022-9026 (2002-01-26)
The inhibitory domains of calpastatin contain three highly conserved regions, A, B, and C, of which A and C bind calpain in a strictly Ca(2+)-dependent manner but have no inhibitory activity whereas region B inhibits calpain on its own. We
BmATG5 and BmATG6 mediate apoptosis following autophagy induced by 20-hydroxyecdysone or starvation.
Kun Xie et al.
Autophagy, 12(2), 381-396 (2016-01-05)
Autophagy and apoptosis, which could be induced by common stimuli, play crucial roles in development and disease. The functional relationship between autophagy and apoptosis is complex, due to the dual effects of autophagy. In the Bombyx Bm-12 cells, 20-hydroxyecdysone (20E)
Zhenping Chen et al.
FASEB bioAdvances, 1(3), 151-166 (2018-12-03)
Endogenous fragments of p53 protein were identified in human cytomegalovirus (HCMV)-infected human lung fibroblasts, particularly a 44-kDa N-terminal fragment [hereafter referred to as p53(ΔCp44)], generated via calpain cleavage. The fragment abundance increased in a biphasic manner, peaking at 6-9 hours and
Bruno Becker et al.
Molecular neurodegeneration, 13(1), 47-47 (2018-08-31)
Neurogranin (Ng) is a small 7.6 kDa postsynaptic protein that has been detected at elevated concentrations in cerebrospinal fluid (CSF) of patients with Alzheimer's disease (AD), both as a full-length molecule and as fragments from its C-terminal half. Ng is involved
Courtney Blachford et al.
Cell calcium, 46(4), 257-262 (2009-09-08)
Neuronal calcium sensor-1 (NCS-1) is a high-affinity, low-capacity Ca(2+)-binding protein expressed in many cell types. We previously showed that NCS-1 interacts with inositol 1,4,5-trisphosphate receptor (InsP(3)R) and modulates Ca(2+)-signaling by enhancing InsP3-dependent InsP(3)R channel activity and intracellular Ca(2+) transients. Recently
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service