All Photos(1)
About This Item
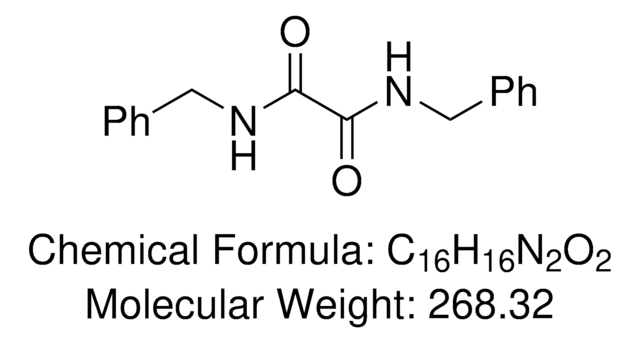
Empirical Formula (Hill Notation):
C16H16N2O2
CAS Number:
Molecular Weight:
268.31
MDL number:
UNSPSC Code:
12352101
NACRES:
NA.21
Recommended Products
Quality Level
Assay
≥95%
form
powder
SMILES string
O=C(C(NCC1=CC=CC=C1)=O)NCC2=CC=CC=C2
InChI
1S/C16H16N2O2/c19-15(17-11-13-7-3-1-4-8-13)16(20)18-12-14-9-5-2-6-10-14/h1-10H,11-12H2,(H,17,19)(H,18,20)
InChI key
AOIKOYRULAZZLJ-UHFFFAOYSA-N
Application
DBO is a ligand for the copper-catalyzed couplings of heteroanilines with (hetero)aryl halides.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Zhixiang Chen et al.
Organic letters, 21(17), 6874-6878 (2019-08-16)
N,N'-Dibenzyloxalamide (DBO) was identified as a powerful ligand for promoting Cu-catalyzed coupling of heteroanilines with (hetero)aryl halides. For (hetero)aryl chlorides, the coupling reaction occurred at 130 °C with 5 mol % CuBr and 10 mol % DBO. For (hetero)aryl bromides/iodides
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service