All Photos(1)
About This Item
Empirical Formula (Hill Notation):
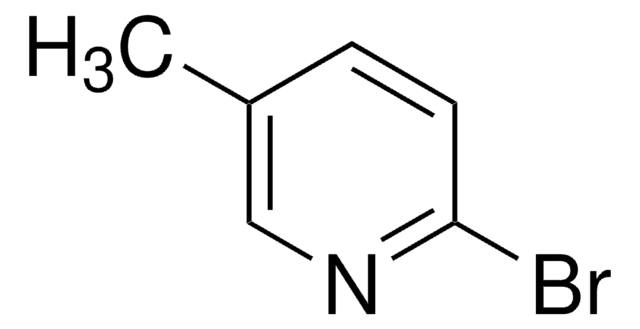
C6H6BrN
CAS Number:
Molecular Weight:
172.02
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
refractive index
n20/D 1.56 (lit.)
bp
199-200 °C (lit.)
density
1.549 g/mL at 25 °C (lit.)
SMILES string
Cc1ccncc1Br
InChI
1S/C6H6BrN/c1-5-2-3-8-4-6(5)7/h2-4H,1H3
InChI key
GSQZOLXWFQQJHJ-UHFFFAOYSA-N
Application
3-Bromo-4-methylpyridine may be used as a building block in the preparation of:
- substituted 4-(2,2-diphenylethyl)pyridine-N-oxides for use as potent phosphodiesterase type 4 (PDE4) inhibitors
- benzodiazepine site ligands bearing tricyclic pyridone moiety for human GABAA receptor
- a novel isomer of ascididemin
- 3-bromopyridine-4-carbonitrile
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
174.9 °F - closed cup
Flash Point(C)
79.4 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
James Crawforth et al.
Bioorganic & medicinal chemistry letters, 14(7), 1679-1682 (2004-03-18)
A series of tricyclic pyridones has been evaluated as benzodiazepine site ligands with functional selectivity for the alpha(3) over the alpha(1) containing subtype of the human GABA(A) receptor ion channel. This investigation led to the identification of a high affinity
Richard Frenette et al.
Bioorganic & medicinal chemistry letters, 12(20), 3009-3013 (2002-09-25)
A detailed SAR study directed toward the optimization of pharmacokinetic parameters for analogues of L-791,943 is reported. The introduction of a soft metabolic site on this structure permitted the identification of L-826,141 as a potent phosphodiesterase type 4 (PDE4) inhibitor
Condensed heteroaromatic ring systems. XV. Synthesis of pyranopyridinones from halopyridinecarbonitriles.
Sakamoto T, et al.
Chemical & Pharmaceutical Bulletin, 36(5), 1890-1894 (1988)
Ida Nymann Petersen et al.
Chemical communications (Cambridge, England), 48(72), 9092-9094 (2012-08-07)
A new and convergent synthesis of ascididemin is presented. Using an anionic cascade ring closure as the key step, this natural product is obtained in 45% overall yield in just 6 steps starting from 2'-fluoroacetophenone. This new approach was extended
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service