All Photos(1)
About This Item
Empirical Formula (Hill Notation):
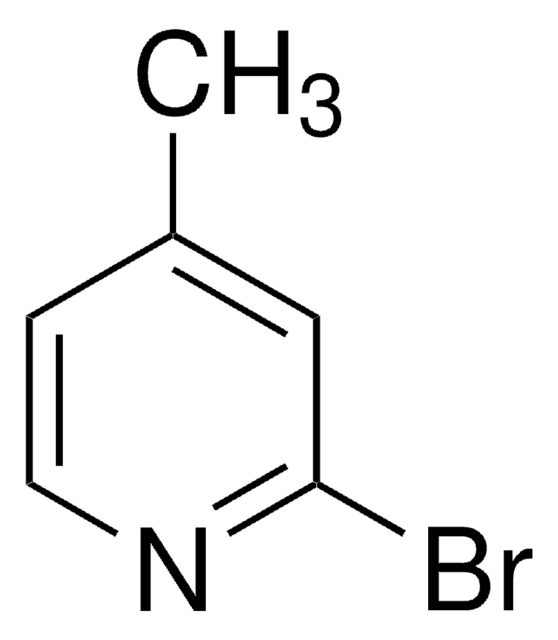
C6H6BrNO
CAS Number:
Molecular Weight:
188.02
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.559 (lit.)
bp
206 °C (lit.)
density
1.53 g/mL at 25 °C (lit.)
SMILES string
COc1cccc(Br)n1
InChI
1S/C6H6BrNO/c1-9-6-4-2-3-5(7)8-6/h2-4H,1H3
InChI key
KMODISUYWZPVGV-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
219.9 °F - closed cup
Flash Point(C)
104.4 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Robert T. Jonas et al.
Inorganic chemistry, 37(26), 6615-6629 (2001-10-24)
An efficient modular protocol for synthesizing a series of facial-capping tris-pyridyl ligands, based on the tris(2-pyridyl)methoxymethane backbone, has been developed which allows for systematic variations of the steric demands at the periphery of the ligand. The coordination chemistry of one
Synthesis of 2, 2'-Bipyridines: Versatile Building Blocks for Sexy Architectures and Functional Nanomaterials.
Newkome GR, et al.
European Journal of Organic Chemistry, 2, 235-254 (2004)
A convenient synthesis of cyclopenta [b] pyridin-2, 5-dione as a non-glycosidic cardiotonic agent.
Robert N, et al.
ARKIVOC (Gainesville, FL, United States), 7, 92-100 (2008)
Mohammed K Elmkaddem et al.
Chemical communications (Cambridge, England), 46(6), 925-927 (2010-01-29)
A copper(i) catalyzed amination reaction utilizing aqueous ammonia and operating under mild conditions is presented. This method was employed for the efficient synthesis of various aminopyridine derivatives bearing electron withdrawing and electron donating groups.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)




