All Photos(1)
About This Item
Linear Formula:
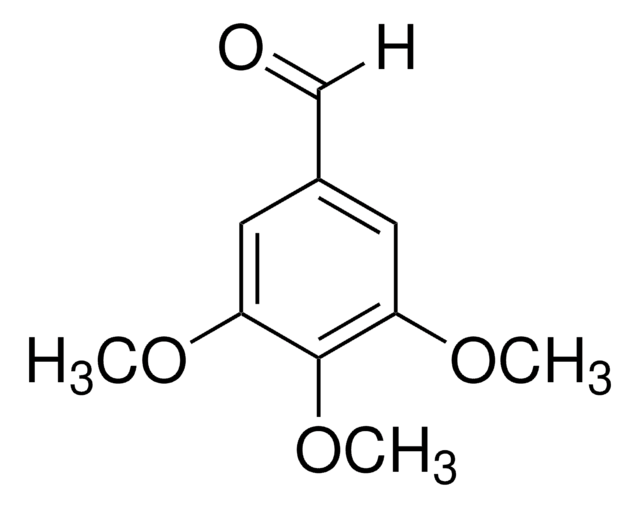
(CH3O)3C6H2CO2H
CAS Number:
Molecular Weight:
212.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98%
form
solid
mp
99-102 °C (lit.)
functional group
carboxylic acid
SMILES string
COc1ccc(C(O)=O)c(OC)c1OC
InChI
1S/C10H12O5/c1-13-7-5-4-6(10(11)12)8(14-2)9(7)15-3/h4-5H,1-3H3,(H,11,12)
InChI key
HZNQSWJZTWOTKM-UHFFFAOYSA-N
Application
2,3,4-Trimethoxybenzoic acid was used in the synthesis of tropoloisoquinoline alkaloid pareitropone. It was also used in the synthesis of isomeric tris(pyrogallol) derivatives and naphthoic acid.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis and characterization of a series of vanadium-tunichrome B 1 analogs. Crystal structure of a tris (catecholamide) complex of vanadium.
Bulls AR, et al.
Journal of the American Chemical Society, 112(7), 2627-2632 (1990)
Hung-Sheng Soung et al.
Neurotoxicity research, 34(3), 375-387 (2018-04-10)
Reserpine (RES)-induced orofacial dyskinesia (OD) has been used as an animal model for human tardive dyskinesia (TD) for decades, due to its strong pathophysiological association with striatal oxidative stress and neural cytoarchitecture alteration. L-Theanine (LT), one of the major amino
Ken S Feldman et al.
Journal of the American Chemical Society, 124(39), 11600-11601 (2002-09-26)
The synthesis of the tropoloisoquinoline alkaloid pareitropone has been accomplished in 14 steps from 2,3,4-trimethoxybenzoic acid. The key transformations include the generation of an alkylidenecarbene intermediate through intramolecular addition of a tosylamide anion to an alkynyliodonium salt, and the cycloaddition
An oxazoline based approach to (S)-Gossypol.
Meyers AI and Willemsen JJ.
Tetrahedron, 54(35), 10493-10511 (1998)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)