M6574
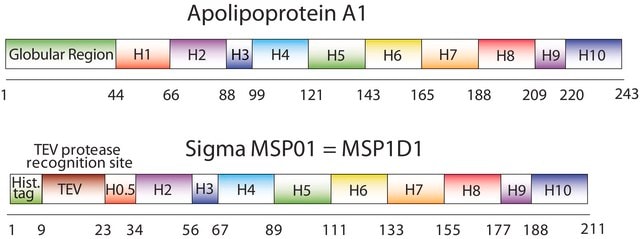
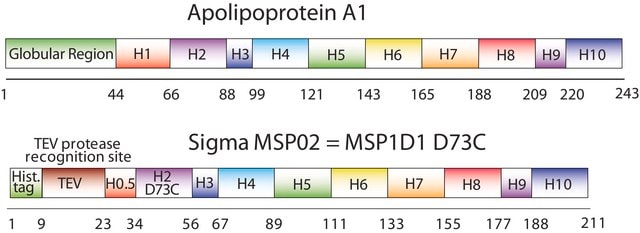
Membrane Scaffold Protein 1D1
recombinant, expressed in E. coli
Synonym(s):
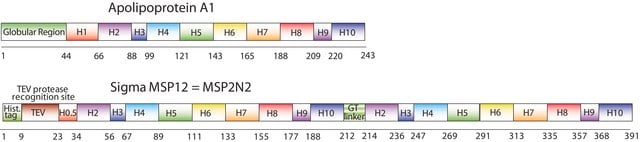
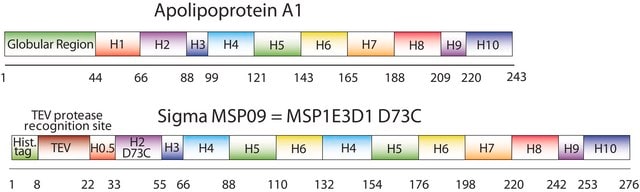
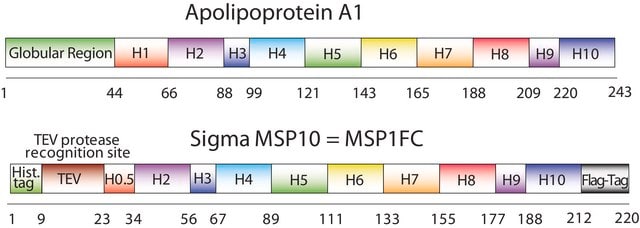
MSP1D1, MSP1T2
About This Item
Recommended Products
biological source
microbial
Quality Level
recombinant
expressed in E. coli
description
N-Terminal histidine-tagged
form
lyophilized powder
mol wt
Mw 24661.9 by amino acid sequence
ε (extinction coefficient)
18200 M-1cm-1 at 280 nm (His-tag-cleaved dissolved in 20 mM Tris pH 7.4, 0.1M NaCl, 0.5mM EDTA and 0.01%NaN3)(lit.)
21000 M-1cm-1 at 280 nm (uncleaved His-tagged dissolved in 20 mM Tris pH 7.4, 0.1M NaCl, 0.5mM EDTA and 0.01%NaN3)(lit.)
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
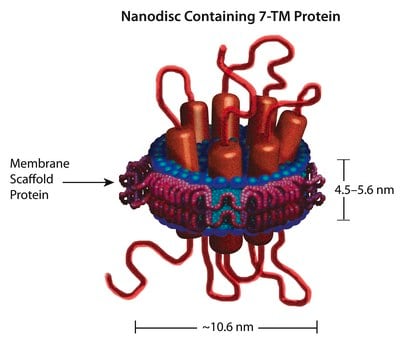
Application
Biochem/physiol Actions
Physical properties
Physical form
Legal Information
- 7,691,414 Membrane scaffold proteins
- 7,662,410 Membrane scaffold proteins and embedded membrane proteins
- 7,622,437 Tissue factor compositions and methods
- 7,592,008 Membrane scaffold proteins
- 7,575,763 Membrane scaffold proteins and tethered membrane proteins
- 7,083,958 Membrane scaffold proteins
- 7,048,949 Membrane scaffold proteins
related product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service