D3168
Duramycin from Streptoverticillium cinnamoneus
≥90.0%
Synonym(s):
Duramycin, Lancovutide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
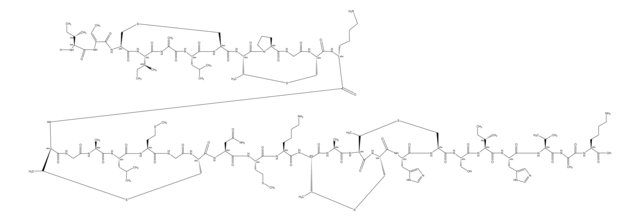
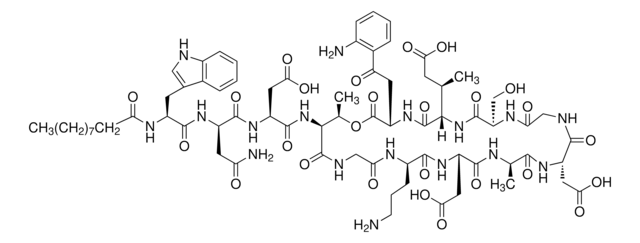
C89H125N23O25S3
CAS Number:
Molecular Weight:
2013.28
MDL number:
UNSPSC Code:
51102829
NACRES:
NA.85
Recommended Products
Quality Level
Assay
≥90.0%
form
powder
solubility
0.1 M HCl: 10 mg/mL
antibiotic activity spectrum
Gram-negative bacteria
Gram-positive bacteria
Mode of action
protein synthesis | interferes
storage temp.
2-8°C
General description
Chemical structure: tetracycline
Application
Duramycin is used to study chloride secretion by cardial, pancreatic and airway epithelial voltage-gated ion channels. It is used to study cystic fibrosis and as a novel phosphatidylethanolamine-binding molecular probe. It is used to stimulate sodium transport in cultured human colonic epithelia.
Biochem/physiol Actions
Duramycin is the smallest known polypeptide since it has only 19 amino acids. It has a defined 3-dimensional binding structure. Duramycin binds phosphatidylethanolamine (PtdE) at a 1:1 ratio with high affinity and exclusive specificity. It enhances chloride secretion in airway epithelium.
Other Notes
Keep container tightly closed in a dry and well-ventilated place.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Soichiro Matsunaga et al.
Langmuir : the ACS journal of surfaces and colloids, 25(14), 8200-8207 (2009-05-13)
We visualized nanometer-scale phospholipid particle fusion by scanning tunneling microscopy (STM) on an alkanethiol-modified gold substrate, induced by duramycin, a tetracyclic antibiotic peptide with 19 amino residues. Ultrasonic homogenization generated a suspension mainly consisting of minimal lipid particles (MLP) from
Ming Zhao et al.
Journal of nuclear medicine : official publication, Society of Nuclear Medicine, 49(8), 1345-1352 (2008-07-18)
With only 19 amino acids, duramycin is the smallest known polypeptide that has a defined 3-dimensional binding structure. Duramycin binds phosphatidylethanolamine (PtdE) at a 1:1 ratio with high affinity and exclusive specificity. As an abundant binding target, PtdE is a
Ilka Steiner et al.
Naunyn-Schmiedeberg's archives of pharmacology, 378(3), 323-333 (2008-05-27)
Duramycin (Moli1901) is being developed for the treatment of reduced mucociliary clearance in cystic fibrosis. This study was conducted to estimate lung residence time and systemic exposure and to assess whether duramycin causes an inflammatory response. Six volunteers were administered
Jason H Stafford et al.
Neoplasia (New York, N.Y.), 13(4), 299-308 (2011-04-08)
We have previously shown that oxidative stress within the tumor microenvironment causes phosphatidylserine (PS) to redistribute from the inner to the outer membrane leaflet of the endothelial cells (EC) creating a highly specific marker for the tumor vasculature. Because the
Marijke De Saint-Hubert et al.
Methods (San Diego, Calif.), 48(2), 178-187 (2009-04-14)
Apoptosis (programmed cell death) and necrosis (uncontrolled cell death) are two distinct processes of cell death that have been described. Non-invasive molecular imaging of these two processes can have several clinical applications and has various approaches in pre-clinical research. Apoptosis
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service