C-12974
Human Mesenchymal Stem Cells (hMSC)
Derived from bone marrow, 500,000 cryopreserved cells
Synonym(s):
Mesenchymal Stem Cell Culture
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
41106514
NACRES:
NA.81
Recommended Products
biological source
human bone marrow
packaging
pkg of 500,000 cells
morphology
(stromal)
technique(s)
cell culture | mammalian: suitable
shipped in
dry ice
storage temp.
−196°C
General description
Lot specific orders are not able to be placed through the web. Contact your local sales rep for more details.
Cell Line Origin
Bone Marrow
Application
Mesenchymal Stem Cells (MSC), also termed Mesenchymal Stromal Cells, are multipotent cells that can differentiate into a variety of cell types and have the capacity for self renewal. MSC have been shown to differentiate in vitro or in vivo into adipocytes, chondrocytes, osteoblasts, myocytes, neurons, hepatocytes, and pancreatic islet cells. Optimized PromoCell media are available to support both the growth of MSC and their differentiation into several different lineages. Recent experiments suggest that differentiation capabilities into diverse cell types vary between MSC of different origin.Human Mesenchymal Stem Cells (hMSC-BM) are harvested from normal human bone marrow from individual donors and are provided in a cryopreserved format. The cells are tested for their ability to differentiate in vitro into adipocytes, chondrocytes, and osteoblasts. The cells show a verified marker expression profile that complies with ISCT recommendations, providing well characterized cells (Cytotherapy (2006) Vol. 8, No. 4, 315-317).
Quality
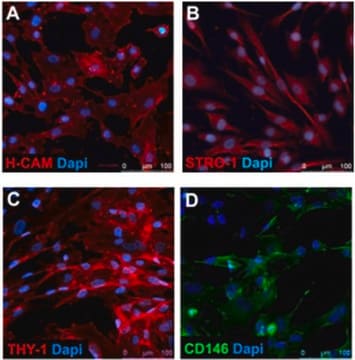
Rigid quality control tests are performed for each lot of Mesenchymal Stem Cells. They are tested for cell morphology, proliferation potential, adherence rate, and viability. Furthermore, they are characterized by flow cytometric analysis of a comprehensive panel of markers, namely CD73/CD90/CD105 and CD14/CD19/CD34/CD45/HLA-DR as proposed by the ISCT. Differentiation assays into adipogenic, osteogenic and chrondrogenic are performed for each lot under culture conditions without antibiotics and antimycotics. In addition, all cells have been tested for the absence of HIV-1, HIV-2, HBV, HCV, HTLV-1 and HTLV-2, and microbial contaminants (fungi, bacteria, and mycoplasma).
Warning
Although tested negative for HIV-1, HIV-2, HBV, HCV, HTLV-1 and HTLV-2, the cells – like all products of human origin – should be handled as potentially infectious. No test procedure can completely guarantee the absence of infectious agents.
Subculture Routine
Click here for more information.
Other Notes
Recommended Plating Density: 4000 cells per cm2Tested Markers: CD105 positive, CD73 positive, CD90 positive, CD45 negative, CD34 negative, CD14 negative, CD19 negative, HLA-DR negativeDifferentiation capacity to adipogenic, osteogenic and chondrogenic cells
Recommended products
Recommended Primary Cell Culture Media:Link
Recommended Primary Cell Culture Media:Link
Disclaimer
RESEARCH USE ONLY. This product is regulated in France when intended to be used for scientific purposes, including for import and export activities (Article L 1211-1 paragraph 2 of the Public Health Code). The purchaser (i.e. enduser) is required to obtain an import authorization from the France Ministry of Research referred in the Article L1245-5-1 II. of Public Health Code. By ordering this product, you are confirming that you have obtained the proper import authorization.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Francesca Gamna et al.
Nanomaterials (Basel, Switzerland), 13(3) (2023-02-12)
The main unmet medical need of bone implants is multifunctional activity, including their ability to induce rapid and physiological osseointegration, counteract bacterial biofilm formation, and prevent in situ chronic inflammation at the same time. This research starts from an already
Stefano Gabetti et al.
Scientific reports, 12(1), 13859-13859 (2022-08-17)
In bone tissue engineering research, bioreactors designed for replicating the main features of the complex native environment represent powerful investigation tools. Moreover, when equipped with automation, their use allows reducing user intervention and dependence, increasing reproducibility and the overall quality
Mehri Sohrabi et al.
Biomedicines, 8(12) (2020-12-19)
Bioactive glass (BG) represents a promising biomaterial for bone healing; here injectable BG pastes biological properties were improved by the addition of gelatin or chitosan, as well as mechanical resistance was enhanced by adding 10 or 20 wt% 3-Glycidyloxypropyl trimethoxysilane
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service