A2419
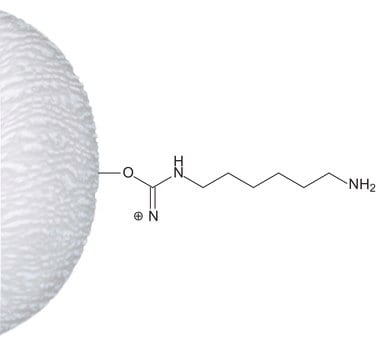
ω-Aminohexyl–Sepharose™ 4B
aqueous ethanol suspension
Synonym(s):
ψ-Aminohexyl Resin, Aminohexyl Sepharose
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Recommended Products
form
aqueous ethanol suspension
Quality Level
technique(s)
affinity chromatography: suitable
matrix activation
epoxy
matrix attachment
amino
matrix spacer
11 atoms
storage temp.
2-8°C
Application
ω-Aminohexyl– Sepharose™ 4B is used mainly in affinity chromatography. It has been used in mitochondrial research to purify thioredoxin reductase (TrxR2), which is important in cell signaling and may also be an anticancer drug target.
Physical form
Suspension in 20% ethanol.
Legal Information
Sepharose is a trademark of Cytiva
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
95.0 °F
Flash Point(C)
35 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sabine Urig et al.
Seminars in cancer biology, 16(6), 452-465 (2006-10-24)
Thioredoxin reductase (TrxR)-as part of a major thiol regulating system-allows redox metabolism to adjust to cellular requirements. Therefore, changes at the redox level reflect as a pars pro toto changes concerning the entire cell. Three different TrxR isoenzymes, TrxR1 as
M Burgos-Trinidad et al.
The Journal of biological chemistry, 267(5), 3498-3505 (1992-02-15)
The chick kidney mitochondrial cytochrome P-450 1,25-dihydroxyvitamin D3 24-hydroxylase was partially purified by sequential polyethylene glycol precipitation, aminohexyl-Sepharose 4B, and hydroxylapatite chromatography. The specific activity of the final preparation, when reconstituted with NADPH, adrenodoxin, and adrenodoxin reductase, was 245 pmol/min/mg
F Pfennig et al.
The Journal of biological chemistry, 274(18), 12508-12516 (1999-04-23)
Actinomycin synthetase I (ACMS I) activates 4-methyl-3-hydroxyanthranilic acid, the precursor of the chromophoric moiety of the actinomycin, as adenylate. The gene acmA of ACMS I was identified upstream of the genes acmB and acmC encoding the two peptide synthetases ACMS
J D Hayes et al.
The Biochemical journal, 207(3), 459-470 (1982-12-01)
Rat liver glutathione S-transferases have previously been defined by their elution behaviour from DEAE-cellulose and CM-cellulose as M, E, D, C, B, A and AA. These enzymes are dimeric proteins which comprise subunits of mol.wt. 22 000 (Ya), 23 500
R Swanson et al.
The Journal of biological chemistry, 260(2), 1287-1289 (1985-01-25)
Bacterial luciferase from Vibrio harveyi, the 77,000-dalton light-emitting enzyme of the marine bacterium, has been crystallized into a two million cubic Angstrom cell with P212121 symmetry. The cell constants are a = 59.6 +/- 0.4 A, b = 112 +/-
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service