All Photos(1)
About This Item
Empirical Formula (Hill Notation):
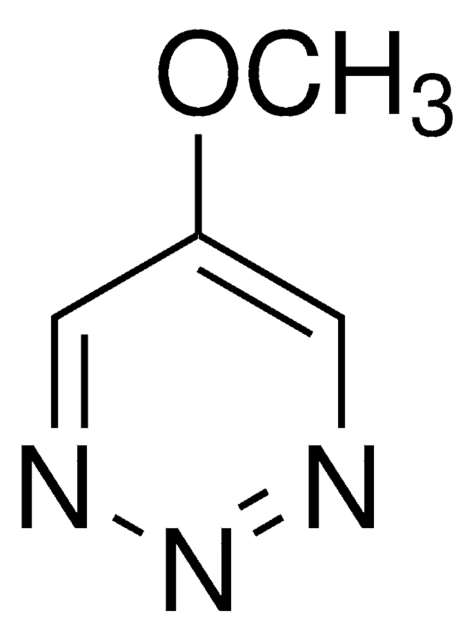
C9H7N3
CAS Number:
Molecular Weight:
157.17
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95%
form
flakes
mp
141-146 °C
storage temp.
−20°C
SMILES string
C1(C2=CC=CC=C2)=CN=NN=C1
InChI
1S/C9H7N3/c1-2-4-8(5-3-1)9-6-10-12-11-7-9/h1-7H
InChI key
KJZQIXWSZPPOHO-UHFFFAOYSA-N
Related Categories
General description
5-Phenyl-1,2,3-triazine is a phenyl triazine derivative. 5-phenyl-1,2,3-triazine exhibits electronic and nonlinear optical properties. 5-Phenyl-1,2,3-triazine can be prepared from 4-bromopyrazole. It undergoes Diels-Alder reaction with ketene acetal.
Application
The following 1,2,3-triazine was reported by Boger and coworkers to undergo an Inverse Electron Demand Diels-Alder with electron rich dienophiles to afford nitrogen-containing heterocycles, more specifically pyrimidines and novel-substituted pyridines.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Investigation of torsional barriers and nonlinear optical (NLO) properties of phenyltriazines.
Alyar H, et al.
Journal of Molecular Structure, 834, 516-520 (2007)
Erin D Anderson et al.
Journal of the American Chemical Society, 133(31), 12285-12292 (2011-07-09)
A systematic study of the inverse electron demand Diels-Alder reactions of 1,2,3-triazines is disclosed, including an examination of the impact of a C5 substituent. Such substituents were found to exhibit a remarkable impact on the cycloaddition reactivity of the 1,2,3-triazine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service