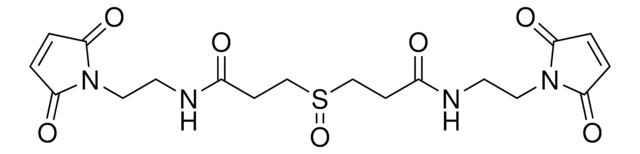
909602
DSSO crosslinker
≥95%
Synonym(s):
Bis(2,5-dioxopyrrolidin-1-yl) 3,3′-sulfinyldipropionate, Bis-(propionic acid NHS ester)-sulfoxide, Mass spectrometry-cleavable crosslinker for studying protein-protein interations
About This Item
Recommended Products
Assay
≥95%
form
powder
availability
available only in USA
storage temp.
2-8°C
Application
Other Notes
Development of a novel cross-linking strategy for fast and accurate identification of cross-linked peptides of protein complexes
Structural dynamics of the human COP9 signalosome revealed by cross-linking mass spectrometry and integrative modeling
Developing a Multiplexed Quantitative Cross-Linking Mass Spectrometry Platform for Comparative Structural Analysis of Protein Complexes
Development of a Novel Sulfoxide-Containing MS-Cleavable Homobifunctional Cysteine-Reactive Cross-Linker for Studying Protein–Protein Interactions
Developing an Acidic Residue Reactive and Sulfoxide-Containing MS-Cleavable Homobifunctional Cross-Linker for Probing Protein-Protein Interactions
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Self-react. C
Storage Class Code
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service