745022
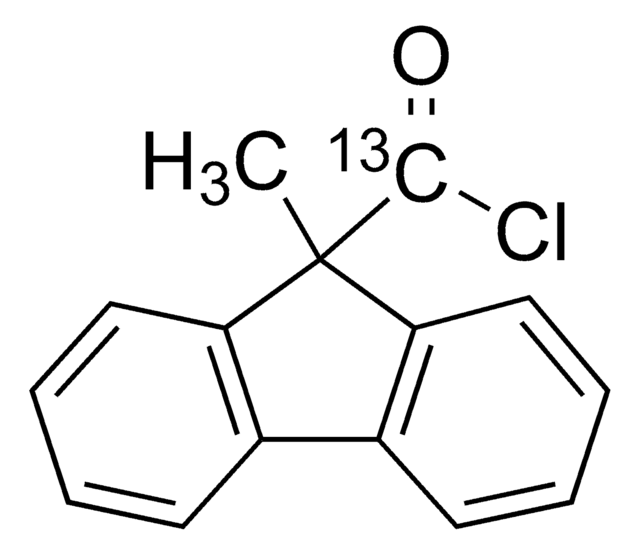
9-Methyl-9H-fluorene-9-carbonyl chloride
≥99.0% (GC)
Synonym(s):
COgen, 9-Methylfluorene-9-carbonyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C15H11ClO
CAS Number:
Molecular Weight:
242.70
Beilstein:
5266487
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥99.0% (GC)
form
solid
reaction suitability
reaction type: C-C Bond Formation
functional group
acyl chloride
SMILES string
CC1(C(Cl)=O)c2ccccc2-c3ccccc13
InChI
1S/C15H11ClO/c1-15(14(16)17)12-8-4-2-6-10(12)11-7-3-5-9-13(11)15/h2-9H,1H3
InChI key
ZQYOOHGEBHBNTP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
COGen is a simple alternative for carbon monoxide generation in the application of palladium-catalyzed carbonylation reactions. This reagent has proved to be applicable in a wide range of applications and is compatible with several building blocks in the formation of ketones, amides, esters, etc.
For more information please visit: Technology Spotlight, Professor Skrystrup PPP
Use with the COware Platform
For more information please visit: Technology Spotlight, Professor Skrystrup PPP
Use with the COware Platform
Linkage
Frequently Asked Questions are available for this Product.
also commonly purchased with this product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
[14C]-Carbon Monoxide
Journal of Labelled Compounds & Radiopharmaceuticals, 55, 411-418 (2012)
Philippe Hermange et al.
Organic letters, 13(9), 2444-2447 (2011-04-08)
A carbonylative Heck reaction of aryl iodides and styrene derivatives employing a two-chamber system using a stable, crystalline, and nontransition metal based carbon monoxide source is reported. By applying near-stoichiometric amounts of the carbon monoxide precursor, an effective exploitation of
Palladium-catalyzed carbonylative α-arylation for accessing 1,3-diketones.
Thomas M Gøgsig et al.
Angewandte Chemie (International ed. in English), 51(3), 798-801 (2011-12-06)
Philippe Hermange et al.
Journal of the American Chemical Society, 133(15), 6061-6071 (2011-03-31)
A new technique for the ex situ generation of carbon monoxide (CO) and its efficient incorporation in palladium catalyzed carbonylation reactions was achieved using a simple sealed two-chamber system. The ex situ generation of CO was derived by a palladium
Mia N Burhardt et al.
The Journal of organic chemistry, 77(12), 5357-5363 (2012-05-23)
We have synthesized two isotopically labeled variants of the β-amyloid binding compound FSB possessing (13)C-labels on the two terminal aryl carboxylic acid moieties. One of these was also fully deuterated on the olefinic spacers. The (13)C-isotope labeling was achieved applying
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service